Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial
- PMID: 18723857
- PMCID: PMC2733178
- DOI: 10.1093/bja/aen248
Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial
Abstract
Background: Relationships between plasma morphine concentrations and neonatal responses to endotracheal tube (ETT) suctioning are unknown in preterm neonates.
Methods: Ventilated preterm neonates (n=898) from 16 centres were randomly assigned to placebo (n=449) or morphine (n=449). After an i.v. loading dose (100 microg kg(-1)), morphine infusions [23-26 weeks postmenstrual age (PMA) 10 microg kg(-1) h(-1); 27-29 weeks 20 microg kg(-1) h(-1); and 30-32 weeks 30 microg kg(-1) h(-1)] were established for a maximum of 14 days. Open-label morphine (20-100 microg kg(-1)) was given for pain or agitation. Morphine assay and neonatal response to ETT suctioning was measured at 20-28 and 70-76 h after starting the drug infusion and at 10-14 h after discontinuation of the study drug. The concentration-effect response was investigated using non-linear mixed effects models.
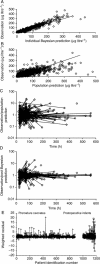
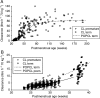
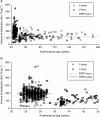
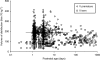
Results: A total of 5119 data points (1598 measured morphine concentrations and 3521 effect measures) were available from 875 neonates for analysis. Clearance was 50% that of the mature value at 54.2 weeks PMA (CLmat(50)) and increased from 2.05 litre h(-1) 70 kg(-1) at 24 weeks PMA to 6.04 litre h(-1) 70 kg(-1) at 32 weeks PMA. The volume of distribution in preterm neonates was 190 litre 70 kg(-1) (CV 51%) and did not change with age. There was no relationship between morphine concentrations (range 0-440 microg litre(-1)) and heart rate changes associated with ETT suctioning or with the Premature Infant Pain Profile.
Conclusions: A sigmoid curve describing maturation of morphine clearance is moved to the right in preterm neonates and volume of distribution is increased compared with term neonates. Morphine does not alter the neonatal response to ETT suctioning.
Figures


References
-
- Chay PC, Duffy BJ, Walker JS. Pharmacokinetic–pharmacodynamic relationships of morphine in neonates. Clin Pharmacol Ther. 1992;51:334–42. - PubMed
-
- Scott CS, Riggs KW, Ling EW, et al. Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr. 1999;135:423–9. - PubMed
-
- Anand KJ, Hall RW, Desai N, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–82. - PubMed
-
- Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208–17. - PubMed
-
- Holford NHG. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30:329–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

