Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin
- PMID: 18724372
- PMCID: PMC2614210
- DOI: 10.1038/ni.1646
Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin
Abstract
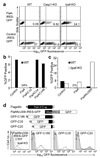
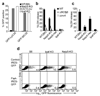
Inflammasomes are cytosolic multiprotein complexes that sense microbial infection and trigger cytokine production and cell death. However, the molecular components of inflammasomes and what they sense remain poorly defined. Here we demonstrate that 35 amino acids of the carboxyl terminus of flagellin triggered inflammasome activation in the absence of bacterial contaminants or secretion systems. To further elucidate the host flagellin-sensing pathway, we generated mice deficient in the intracellular sensor Naip5. These mice failed to activate the inflammasome in response to the 35 amino acids of flagellin or in response to Legionella pneumophila infection. Our data clarify the molecular basis for the cytosolic response to flagellin.
Figures




References
-
- Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. - PubMed
-
- Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. - PubMed
-
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. - PubMed
-
- Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

