The GET complex mediates insertion of tail-anchored proteins into the ER membrane
- PMID: 18724936
- PMCID: PMC2572727
- DOI: 10.1016/j.cell.2008.06.025
The GET complex mediates insertion of tail-anchored proteins into the ER membrane
Abstract
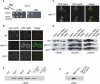
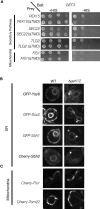
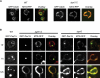
Tail-anchored (TA) proteins, defined by the presence of a single C-terminal transmembrane domain (TMD), play critical roles throughout the secretory pathway and in mitochondria, yet the machinery responsible for their proper membrane insertion remains poorly characterized. Here we show that Get3, the yeast homolog of the TA-interacting factor Asna1/Trc40, specifically recognizes TMDs of TA proteins destined for the secretory pathway. Get3 recognition represents a key decision step, whose loss can lead to misinsertion of TA proteins into mitochondria. Get3-TA protein complexes are recruited for endoplasmic reticulum (ER) membrane insertion by the Get1/Get2 receptor. In vivo, the absence of Get1/Get2 leads to cytosolic aggregation of Get3-TA complexes and broad defects in TA protein biogenesis. In vitro reconstitution demonstrates that the Get proteins directly mediate insertion of newly synthesized TA proteins into ER membranes. Thus, the GET complex represents a critical mechanism for ensuring efficient and accurate targeting of TA proteins.
Figures
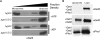


References
-
- Abell B.M., Rabu C., Leznicki P., Young J.C., High S. Post-translational integration of tail-anchored proteins is facilitated by defined molecular chaperones. J. Cell Sci. 2007;120:1743–1751. - PubMed
-
- Beilharz T., Egan B., Silver P.A., Hofmann K., Lithgow T. Bipartite signals mediate subcellular targeting of tail-anchored membrane proteins in Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:8219–8223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

