Opposing Wnt pathways orient cell polarity during organogenesis
- PMID: 18724937
- PMCID: PMC2603076
- DOI: 10.1016/j.cell.2008.06.026
Opposing Wnt pathways orient cell polarity during organogenesis
Abstract
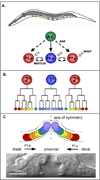
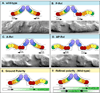
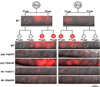
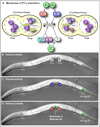
The orientation of asymmetric cell division contributes to the organization of cells within a tissue or organ. For example, mirror-image symmetry of the C. elegans vulva is achieved by the opposite division orientation of the vulval precursor cells (VPCs) flanking the axis of symmetry. We characterized the molecular mechanisms contributing to this division pattern. Wnts MOM-2 and LIN-44 are expressed at the axis of symmetry and orient the VPCs toward the center. These Wnts act via Fz/LIN-17 and Ryk/LIN-18, which control beta-catenin localization and activate gene transcription. In addition, VPCs on both sides of the axis of symmetry possess a uniform underlying "ground" polarity, established by the instructive activity of Wnt/EGL-20. EGL-20 establishes ground polarity via a novel type of signaling involving the Ror receptor tyrosine kinase CAM-1 and the planar cell polarity component Van Gogh/VANG-1. Thus, tissue polarity is determined by the integration of multiple Wnt pathways.
Figures


References
-
- Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–1644. - PubMed
-
- Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. - PubMed
-
- Deshpande R, Inoue T, Priess JR, Hill RJ. lin-17/Frizzled and lin-18 regulate POP-1/TCF-1 localization and cell type specification during C. elegans vulval development. Dev Biol. 2005;278:118–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

