Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni
- PMID: 18725315
- PMCID: PMC4122427
- DOI: 10.1016/j.micinf.2008.07.030
Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni
Abstract
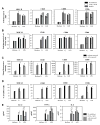
Food-borne Campylobacter jejuni (Cj) is an important cause of enteritis. We showed that C57BL/6 and congenic interleukin (IL)-10(-/-) mice serve as models of Cj colonization and enteritis, respectively. Thus, C57BL/6 mice are resistant to Cj induced disease. Because dendritic cells (DCs) are central to regulating adaptive immune responses, we investigated the interaction of Cj with murine bone marrow-derived DCs (BM-DCs) to assess bacterial killing, DC activation, and the ability of Cj-infected BM-DCs to stimulate Campylobacter-specific T cell responses in vitro. BM-DCs challenged with Cj efficiently internalized and killed Cj 11168 and significantly upregulated surface MHC-II, CD40, CD80 and CD86 demonstrating a mature phenotype. Infected BM-DCs secreted significant amounts of tumor necrosis factor-alpha (TNF-alpha), IL-6 and IL-12p70. Formalin-killed Cj also induced maturation of BM-DCs with similar cytokine production but at a significantly lower magnitude than live bacteria. Maximal activation of murine BM-DCs required internalization of Cj; attachment alone was not sufficient to elicit significant responses. Also, various strains of Cj elicited different magnitudes of cytokine production from BM-DCs. Finally, in a coculture system, Cj-infected BM-DCs induced high level interferon-gamma (INF-gamma) production from CD4+T cells indicating Th1 polarization. Thus, DCs from resistant C57BL/6 mice initiate T cell responses against Cj.
Figures





Similar articles
-
Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy.J Leukoc Biol. 2005 Sep;78(3):656-64. doi: 10.1189/jlb.1104631. Epub 2005 Jun 16. J Leukoc Biol. 2005. PMID: 15961574
-
AIMP1/p43 protein induces the maturation of bone marrow-derived dendritic cells with T helper type 1-polarizing ability.J Immunol. 2008 Mar 1;180(5):2894-902. doi: 10.4049/jimmunol.180.5.2894. J Immunol. 2008. PMID: 18292511
-
Campylobacter jejuni induces maturation and cytokine production in human dendritic cells.Infect Immun. 2006 May;74(5):2697-705. doi: 10.1128/IAI.74.5.2697-2705.2006. Infect Immun. 2006. PMID: 16622206 Free PMC article.
-
Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation.Blood. 2001 Sep 1;98(5):1512-23. doi: 10.1182/blood.v98.5.1512. Blood. 2001. PMID: 11520802
-
Threonyl-tRNA Synthetase Promotes T Helper Type 1 Cell Responses by Inducing Dendritic Cell Maturation and IL-12 Production via an NF-κB Pathway.Front Immunol. 2020 Oct 14;11:571959. doi: 10.3389/fimmu.2020.571959. eCollection 2020. Front Immunol. 2020. PMID: 33178197 Free PMC article.
Cited by
-
Enhanced antigen uptake by dendritic cells induced by the B pentamer of the type II heat-labile enterotoxin LT-IIa requires engagement of TLR2.Vaccine. 2010 May 7;28(21):3696-705. doi: 10.1016/j.vaccine.2010.03.016. Epub 2010 Mar 21. Vaccine. 2010. PMID: 20332049 Free PMC article.
-
Host-specific differences in the response of cultured macrophages to Campylobacter jejuni capsule and O-methyl phosphoramidate mutants.Vet Res. 2018 Jan 9;49(1):3. doi: 10.1186/s13567-017-0501-y. Vet Res. 2018. PMID: 29316981 Free PMC article.
-
The Campylobacter jejuni CiaD effector protein activates MAP kinase signaling pathways and is required for the development of disease.Cell Commun Signal. 2013 Oct 21;11:79. doi: 10.1186/1478-811X-11-79. Cell Commun Signal. 2013. PMID: 24144181 Free PMC article.
-
Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling.PLoS One. 2012;7(7):e40761. doi: 10.1371/journal.pone.0040761. Epub 2012 Jul 10. PLoS One. 2012. PMID: 22808254 Free PMC article.
-
Campylobacter jejuni and associated immune mechanisms: short-term effects and long-term implications for infants in low-income countries.Curr Opin Infect Dis. 2017 Jun;30(3):322-328. doi: 10.1097/QCO.0000000000000364. Curr Opin Infect Dis. 2017. PMID: 28157786 Free PMC article. Review.
References
-
- Wassenaar TM, Blaser MJ. Pathophysiology of Campylobacter jejuni infections of humans. Microb Infect. 1999;1:1023–1033. - PubMed
-
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

