Endocannabinoids in the retina: from marijuana to neuroprotection
- PMID: 18725316
- PMCID: PMC2584875
- DOI: 10.1016/j.preteyeres.2008.07.002
Endocannabinoids in the retina: from marijuana to neuroprotection
Abstract
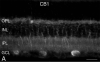
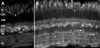
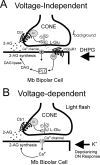
The active component of the marijuana plant Cannabis sativa, Delta9-tetrahydrocannabinol (THC), produces numerous beneficial effects, including analgesia, appetite stimulation and nausea reduction, in addition to its psychotropic effects. THC mimics the action of endogenous fatty acid derivatives, referred to as endocannabinoids. The effects of THC and the endocannabinoids are mediated largely by metabotropic receptors that are distributed throughout the nervous and peripheral organ systems. There is great interest in endocannabinoids for their role in neuroplasticity as well as for therapeutic use in numerous conditions, including pain, stroke, cancer, obesity, osteoporosis, fertility, neurodegenerative diseases, multiple sclerosis, glaucoma and inflammatory diseases, among others. However, there has been relatively far less research on this topic in the eye and retina compared with the brain and other organ systems. The purpose of this review is to introduce the "cannabinergic" field to the retinal community. All of the fundamental works on cannabinoids have been performed in non-retinal preparations, necessitating extensive dependence on this literature for background. Happily, the retinal cannabinoid system has much in common with other regions of the central nervous system. For example, there is general agreement that cannabinoids suppress dopamine release and presynaptically reduce transmitter release from cones and bipolar cells. How these effects relate to light and dark adaptations, receptive field formation, temporal properties of ganglion cells or visual perception are unknown. The presence of multiple endocannabinoids, degradative enzymes with their bioactive metabolites, and receptors provides a broad spectrum of opportunities for basic research and to identify targets for therapeutic application to retinal diseases.
Figures









Similar articles
-
Role of endocannabinoid system in mental diseases.Neurotox Res. 2004;6(3):213-24. doi: 10.1007/BF03033223. Neurotox Res. 2004. PMID: 15325960 Review.
-
The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications.Neural Plast. 2016;2016:2916732. doi: 10.1155/2016/2916732. Epub 2016 Jan 6. Neural Plast. 2016. PMID: 26881099 Free PMC article. Review.
-
Implication of cannabinoids in neurological diseases.Cell Mol Neurobiol. 2006 Jul-Aug;26(4-6):579-91. doi: 10.1007/s10571-006-9070-8. Epub 2006 May 12. Cell Mol Neurobiol. 2006. PMID: 16699878 Free PMC article. Review.
-
Pharmacology and toxicology of Cannabis derivatives and endocannabinoid agonists.Recent Pat CNS Drug Discov. 2010 Jan;5(1):46-52. doi: 10.2174/157488910789753521. Recent Pat CNS Drug Discov. 2010. PMID: 19832688 Review.
-
Cannabinoids and the gut: new developments and emerging concepts.Pharmacol Ther. 2010 Apr;126(1):21-38. doi: 10.1016/j.pharmthera.2009.12.005. Epub 2010 Feb 1. Pharmacol Ther. 2010. PMID: 20117132 Review.
Cited by
-
The intraocular pressure-lowering properties of intravenous paracetamol.Clin Ophthalmol. 2016 Jul 13;10:1283-9. doi: 10.2147/OPTH.S87988. eCollection 2016. Clin Ophthalmol. 2016. PMID: 27471373 Free PMC article.
-
Receptor targets of amacrine cells.Vis Neurosci. 2012 Jan;29(1):11-29. doi: 10.1017/S0952523812000028. Vis Neurosci. 2012. PMID: 22310370 Free PMC article. Review.
-
The Vertical and Horizontal Pathways in the Monkey Retina Are Modulated by Typical and Atypical Cannabinoid Receptors.Cells. 2021 Nov 13;10(11):3160. doi: 10.3390/cells10113160. Cells. 2021. PMID: 34831383 Free PMC article. Review.
-
Presence and regulation of cannabinoid receptors in human retinal pigment epithelial cells.Mol Vis. 2009 Jun 14;15:1243-51. Mol Vis. 2009. PMID: 19547718 Free PMC article.
-
A positive feedback synapse from retinal horizontal cells to cone photoreceptors.PLoS Biol. 2011 May;9(5):e1001057. doi: 10.1371/journal.pbio.1001057. Epub 2011 May 3. PLoS Biol. 2011. PMID: 21559323 Free PMC article.
References
-
- Adams AJ, Brown B. Alcohol prolongs time course of glare recovery. Nature. 1975;257:481–483. - PubMed
-
- Adams AJ, Brown B, Flom MC, Jampolsky A. Alcohol and marijuana effects on static visual acuity. Am. J. Opt. Physiol. Optics. 1975;52:729–735. - PubMed
-
- Adams AJ, Brown B, Haegerstrom-Portnoy G, Flom MC, Jones RT. Marijuana, alcohol, and combined drug effects on the time course of glare recovery. Psychopharmacology (Berl) 1978;56:81–86. - PubMed
-
- Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, Lutz B, Guzmán M, Galve-Roperh I. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J. Biol. Chem. 2007;282:23892–23898. - PubMed
-
- Ahmad AS, Zhuang H, Echeverria V, Doré S. Stimulation of prostaglandin EP2 receptors prevents NMDA-induced excitotoxicity. J. Neurotrauma. 2006;23:1895–1903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

