Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes
- PMID: 18725356
- PMCID: PMC2535678
- DOI: 10.1534/genetics.108.090399
Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes
Abstract
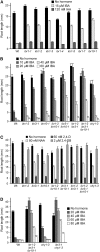
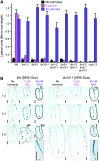
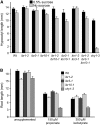
Genetic evidence suggests that indole-3-butyric acid (IBA) is converted to the active auxin indole-3-acetic acid (IAA) by removal of two side-chain methylene units in a process similar to fatty acid beta-oxidation. Previous studies implicate peroxisomes as the site of IBA metabolism, although the enzymes that act in this process are still being identified. Here, we describe two IBA-response mutants, ibr1 and ibr10. Like the previously described ibr3 mutant, which disrupts a putative peroxisomal acyl-CoA oxidase/dehydrogenase, ibr1 and ibr10 display normal IAA responses and defective IBA responses. These defects include reduced root elongation inhibition, decreased lateral root initiation, and reduced IBA-responsive gene expression. However, peroxisomal energy-generating pathways necessary during early seedling development are unaffected in the mutants. Positional cloning of the genes responsible for the mutant defects reveals that IBR1 encodes a member of the short-chain dehydrogenase/reductase family and that IBR10 resembles enoyl-CoA hydratases/isomerases. Both enzymes contain C-terminal peroxisomal-targeting signals, consistent with IBA metabolism occurring in peroxisomes. We present a model in which IBR3, IBR10, and IBR1 may act sequentially in peroxisomal IBA beta-oxidation to IAA.
Figures





References
-
- Adham, A. R., B. K. Zolman, A. Millius and B. Bartel, 2005. Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in β-oxidation. Plant J. 41 859–874. - PubMed
-
- Agnihotri, G., and H.-W. Liu, 2003. Enoyl-CoA hydratase: reaction, mechanism, and inhibition. Bioorg. Med. Chem. 11 9–20. - PubMed
-
- Alonso, J. M., A. N. Stepanova, T. J. Leisse, C. J. Kim, H. Chen et al., 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Arai, Y., M. Hayashi and M. Nishimura, 2008. Proteomic analysis of highly purified peroxisomes from etiolated soybean cotyledons. Plant Cell Physiol. 49 526–539. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman et al., 1999. Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-Interscience, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

