Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma
- PMID: 18725522
- PMCID: PMC2526206
- DOI: 10.1084/jem.20080099
Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma
Abstract
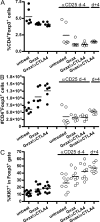
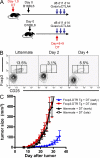
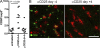
Interference with inhibitory immunological checkpoints controlling T cell activation provides new opportunities to augment cancer immunotherapies. Whereas cytotoxic T lymphocyte-associated antigen-4 blockade has shown promising preclinical and clinical results, therapeutic CD4(+)CD25(+) T reg cell depletion has failed to consistently enhance immune-based therapies. Using B16/BL6, a transplantable murine melanoma model, we show a dichotomy between the effects of T reg cell depletion on tumor rejection dependent on whether depletion occurs before (prophylactic) or after (therapeutic) tumor engraftment. Failure to promote rejection with therapeutic depletion is not related to lack of T reg cell depletion, to elimination of CD25(+) effector T cells, or to a failure to enhance systemic antitumor T cell responses, but correlates with failure of effector cells to infiltrate the tumor and increase the intratumor ratio of effector T cell/T reg cell. Finally, systemic antitumor responses generated upon therapeutic T reg cell depletion are significantly stronger than those generated in the presence of T reg cells, and are capable of eliciting rejection of established tumors after transfer into immunoablated recipients receiving combination immunotherapy. The data demonstrate a dissociation between measurable systemic responses and tumor rejection during CD25-directed T reg cell depletion, and suggest an alternative, clinically applicable strategy for the treatment of established tumors.
Figures





References
-
- Kim, J.M., J.P. Rasmussen, and A.Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197. - PubMed
-
- Wan, Y.Y., and R.A. Flavell. 2007. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 445:766–770. - PubMed
-
- Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. - PubMed
-
- Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

