The critical role of partially exposed N-terminal valine residue in stabilizing GH10 xylanase from Bacillus sp.NG-27 under poly-extreme conditions
- PMID: 18725971
- PMCID: PMC2516601
- DOI: 10.1371/journal.pone.0003063
The critical role of partially exposed N-terminal valine residue in stabilizing GH10 xylanase from Bacillus sp.NG-27 under poly-extreme conditions
Erratum in
- PLoS ONE.2009;4(2). doi: 10.1371/annotation/aefbf6a4-298d-4173-907f-9c9a996249b2.. Bharadwaj, Amit [corrected to Bhardwaj, Amit] doi: 10.1371/annotation/aefbf6a4-298d-4173-907f-9c9a996249b2.
Abstract
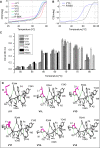
Background: Understanding the mechanisms that govern protein stability under poly-extreme conditions continues to be a major challenge. Xylanase (BSX) from Bacillus sp. NG-27, which has a TIM-barrel structure, shows optimum activity at high temperature and alkaline pH, and is resistant to denaturation by SDS and degradation by proteinase K. A comparative circular dichroism analysis was performed on native BSX and a recombinant BSX (R-BSX) with just one additional methionine resulting from the start codon. The results of this analysis revealed the role of the partially exposed N-terminus in the unfolding of BSX in response to an increase in temperature.
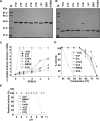
Methodology: We investigated the poly-extremophilicity of BSX to deduce the structural features responsible for its stability under one set of conditions, in order to gain information about its stability in other extreme conditions. To systematically address the role of the partially exposed N-terminus in BSX stability, a series of mutants was generated in which the first hydrophobic residue, valine (Val1), was either deleted or substituted with various amino acids. Each mutant was subsequently analyzed for its thermal, SDS and proteinase K stability in comparison to native BSX.
Conclusions: A single conversion of Val1 to glycine (Gly) changed R-BSX from being thermo- and alkali- stable and proteinase K and SDS resistant, to being thermolabile and proteinase K-, alkali- and SDS- sensitive. This result provided insight into the structure-function relationships of BSX under poly-extreme conditions. Molecular, biochemical and structural data revealed that the poly-extremophilicity of BSX is governed by a partially exposed N-terminus through hydrophobic interactions. Such hitherto unidentified N-terminal hydrophobic interactions may play a similar role in other proteins, especially those with TIM-barrel structures. The results of the present study are therefore of major significance for protein folding and protein engineering.
Conflict of interest statement
Figures



References
-
- Alberti E, Consonni R, Zetta L. Applications of NMR to thermostable proteins. Annual Report in NMR Spectroscopy. 2003;50:121–161.
-
- Matthews BW. Structural and genetic analysis of protein stability. Annu Rev Biochem. 1993;62:139–160. - PubMed
-
- Fersht AR, Serrano L. Principles of protein stability derived from protein engineering experiments. Curr Opin Structuct Biol. 1993;3(1):75–83.
-
- Funahashi J, Tkano K, Yamagata Y, Yutani K. Role of surface hydrophobic residues in the conformational stability of human lysozyme at three diferent positions. Biochemistry. 2000;39(47):14448–14456. - PubMed
-
- Pakula AA, Sauer RT. Reverse hydrophobic effects relieved by amino-acid substitutions at a protein surface. Nature. 1990;344(6264):363–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

