The elusive physiologic role of Factor XII
- PMID: 18725991
- PMCID: PMC2518076
- DOI: 10.1172/JCI36617
The elusive physiologic role of Factor XII
Abstract
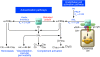
Physiologic hemostasis upon injury involves many plasma proteins in a well-regulated cascade of proteolytic reactions to form a clot. Deficiency of blood coagulation Factors VIII, IX, or XI is associated with hemophilia. Factor XII (FXII) autoactivates by contact with a variety of artificial or biologic negatively charged surfaces (contact activation), resulting in blood coagulation and activation of the inflammatory kallikrein-kinin and complement systems. However, surprisingly, individuals deficient in FXII rarely suffer from bleeding disorders. Most biologic surfaces that activate FXII become expressed in disease states. Investigators have long searched for physiologic activators of FXII and its role in vivo. In this issue of the JCI, Maas et al. show that misfolded protein aggregates produced during systemic amyloidosis allow for plasma FXIIa and prekallikrein activation and increased formation of kallikrein-C1 inhibitor complexes, without Factor XIa activation and coagulation (see the related article beginning on page 3208). This study describes a novel biologic surface for FXII activation and activity, which initiates inflammatory events independent of hemostasis.
Figures
Comment on
-
Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation.J Clin Invest. 2008 Sep;118(9):3208-18. doi: 10.1172/JCI35424. J Clin Invest. 2008. PMID: 18725990 Free PMC article.
References
-
- Rocha E., Silva M., Beraldo W.T., Rosenfeld G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am. J. Physiol. 1949;156:261–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

