Cotargeting survival signaling pathways in cancer
- PMID: 18725993
- PMCID: PMC2518078
- DOI: 10.1172/JCI36898
Cotargeting survival signaling pathways in cancer
Erratum in
- J Clin Invest. 2008 Oct;118(10):3513
Abstract
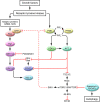
Mammalian target of rapamycin (mTOR) is a component of a signaling pathway (PTEN/PI3K/AKT) that is frequently dysregulated in cancer. However, its precise relationship to the MAPK cascade (Ras/Raf/MEK/ERK), another pathway often implicated in tumorigenesis, has not been well defined. Recent evidence from tissue specimens obtained from patients who have received mTOR inhibitors suggests that ERK may be activated in response to mTOR interruption. In this issue of the JCI, Waugh Kinkade et al. and Carracedo et al. examine the relationship between these pathways in prostate and breast cancer cell model systems (see the related articles beginning on pages 3051 and 3065, respectively). Their findings suggest a link between inhibition of mTOR and ERK activation, possibly reflecting interruption of a novel negative S6K1-dependent feedback loop. Significantly, both groups observed that simultaneous inhibition of MEK/ERK and mTOR resulted in substantially enhanced antitumor effects both in vitro and in vivo. Together, these findings suggest that concurrent interruption of complementary signaling pathways warrants further investigation in cancer therapy.
Figures
Comment on
-
Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model.J Clin Invest. 2008 Sep;118(9):3051-64. doi: 10.1172/JCI34764. J Clin Invest. 2008. PMID: 18725989 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

