Evaluation of a scaling approach for the bioequivalence of highly variable drugs
- PMID: 18726698
- PMCID: PMC2761698
- DOI: 10.1208/s12248-008-9053-4
Evaluation of a scaling approach for the bioequivalence of highly variable drugs
Erratum in
- AAPS J. 2008 Sep;10(3):480
Abstract
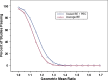
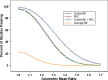
Various approaches for evaluating the bioequivalence (BE) of highly variable drugs (CV > or = 30%) have been debated for many years. More recently, the FDA conducted research to evaluate one such approach: scaled average BE. A main objective of this study was to determine the impact of scaled average BE on study power, and compare it to the method commonly applied currently (average BE). Three-sequence, three period, two treatment partially replicated cross-over BE studies were simulated in S-Plus. Average BE criteria, using 80-125% limits on the 90% confidence intervals for C (max) and AUC geometric mean ratios, as well as scaled average BE were applied to the results. The percent of studies passing BE was determined under different conditions. Variables tested included within subject variability, point estimate constraint, and different values for sigma(w0), which is a constant set by the regulatory agency. The simulation results demonstrated higher study power with scaled average BE, compared to average BE, as within subject variability increased. At 60% CV, study power was more than 90% for scaled average BE, compared with about 22% for average BE. A sigma(w0) value of 0.25 appears to work best. The results of this research project suggest that scaled average BE, using a partial replicate design, is a good approach for the evaluation of BE of highly variable drugs.
Figures




References
-
- U.S. Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations, March 2003.
-
- Schall R., Luus H. G. On Population and Individual Bioequivalence. Stat. Med. 1993;12:1109–1124. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

