NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons
- PMID: 18728191
- PMCID: PMC2525562
- DOI: 10.1073/pnas.0806133105
NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons
Abstract
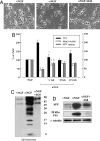
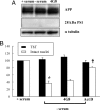
Here, we report that interruption of NGF or BDNF signaling in hippocampal neurons rapidly activates the amyloidogenic pathway and causes neuronal apoptotic death. These events are associated with an early intracellular accumulation of PS1 N-terminal catalytic subunits and of APP C-terminal fragments and a progressive accumulation of intra- and extracellular Abeta aggregates partly released into the culture medium. The released pool of Abeta induces an increase of APP and PS1 holoprotein levels, creating a feed-forward toxic loop that might also cause the death of healthy neurons. These events are mimicked by exogenously added Abeta and are prevented by exposure to beta- and gamma-secretase inhibitors and by antibodies directed against Abeta peptides. The same cultured neurons deprived of serum die, but APP and PS1 overexpression does not occur, Abeta production is undetectable, and cell death is not inhibited by anti-Abeta antibodies, suggesting that hippocampal amyloidogenesis is not a simple consequence of an apoptotic trigger but is due to interruption of neurotrophic signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Allen SJ, Dawbarn D. Clinical relevance of neurotrophins and their receptors. Clin Sci (London) 2006;110:175–191. - PubMed
-
- Williams BJ, Eriksdotter-Jonhagen M, Granholm AC. Nerve growth factor in treatment and pathogenesis of Alzheimer disease. Prog Neurobiol. 2006;80:114–128. - PubMed
-
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. - PubMed
-
- Tuszynski MH, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. - PubMed
-
- Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis. 2007;26:47–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

