Metabolic cycling in control of glucose-stimulated insulin secretion
- PMID: 18728221
- PMCID: PMC2603555
- DOI: 10.1152/ajpendo.90604.2008
Metabolic cycling in control of glucose-stimulated insulin secretion
Abstract
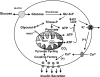
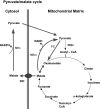
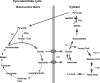
Glucose-stimulated insulin secretion (GSIS) is central to normal control of metabolic fuel homeostasis, and its impairment is a key element of beta-cell failure in type 2 diabetes. Glucose exerts its effects on insulin secretion via its metabolism in beta-cells to generate stimulus/secretion coupling factors, including a rise in the ATP/ADP ratio, which serves to suppress ATP-sensitive K(+) (K(ATP)) channels and activate voltage-gated Ca(2+) channels, leading to stimulation of insulin granule exocytosis. Whereas this K(ATP) channel-dependent mechanism of GSIS has been broadly accepted for more than 30 years, it has become increasingly apparent that it does not fully describe the effects of glucose on insulin secretion. More recent studies have demonstrated an important role for cyclic pathways of pyruvate metabolism in control of insulin secretion. Three cycles occur in islet beta-cells: the pyruvate/malate, pyruvate/citrate, and pyruvate/isocitrate cycles. This review discusses recent work on the role of each of these pathways in control of insulin secretion and builds a case for the particular relevance of byproducts of the pyruvate/isocitrate cycle, NADPH and alpha-ketoglutarate, in control of GSIS.
Figures


References
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPt Boyd 3rd AE, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268: 423–426, 1995. - PubMed
-
- Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB. Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem 273: 16146–16154, 1998. - PubMed
-
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130: 167–178, 1992. - PubMed
-
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312: 446–448, 1984. - PubMed
-
- Assimacopoulos-Jeannet F, Thumelin S, Roche E, Esser V, McGarry JD, Prentki M. Fatty acids rapidly induce the carnitine palmitoyltransferase I gene in the pancreatic beta-cell line INS-1. J Biol Chem 272: 1659–1664, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

