Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1
- PMID: 18728229
- PMCID: PMC2570403
- DOI: 10.2337/db08-0878
Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1
Abstract
Objective: This study was designed to determine whether the transcription factor FoxM1 was required for regeneration of beta-cell mass via proliferation and/or neogenesis in the adult after 60% partial pancreatectomy (PPx).
Research design and methods: Adult mice with a pancreas-wide deletion of Foxm1 (Foxm1(flox/flox);Pdx1-Cre [FoxM1(Deltapanc)]) and their control littermates (Foxm1(flox/flox)) were subjected to PPx or a sham operation, after which islet expression of Foxm1 and several target genes, beta-cell mass, proliferation, beta-cell size, islet size, islet density, and neurogenin-3 expression were analyzed.
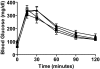
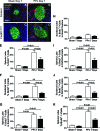
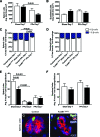
Results: In control mice, PPx stimulated beta-cell proliferation and neogenesis and upregulated Foxm1 and several of its known targets (Plk1, Cenp-a, Birc5/Survivin, and Ccnb1) in islets. Within 1 week post-PPx, control mice underwent significant regeneration of beta-cell mass, and average islet size within the regenerating lobe was similar to that after a sham operation. However, FoxM1(Deltapanc) mice exhibited specific impairments in beta-cell mass regeneration and islet growth after PPx, with reduced proliferation of alpha- and beta-cells but no impairments in acinar or ductal cell proliferation. Interestingly, FoxM1 was not required for proliferation of beta-cells within small endocrine cell clusters located in the regenerating portion of the pancreas but was specifically required for proliferation of beta-cells within larger islets. Additionally, FoxM1 was not required for beta-cell neogenesis following PPx.
Conclusions: Our results indicate that FoxM1 is partially required for increased beta-cell proliferation, but not beta-cell neogenesis, stimulated by PPx. Furthermore, FoxM1 seems to be dispensable for proliferation of beta-cells following neogenesis but is required for proliferation of preexisting beta-cells.
Figures




Similar articles
-
Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen.Diabetes. 2010 Jan;59(1):143-52. doi: 10.2337/db09-0050. Epub 2009 Oct 15. Diabetes. 2010. PMID: 19833884 Free PMC article.
-
The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass.Mol Endocrinol. 2006 Aug;20(8):1853-66. doi: 10.1210/me.2006-0056. Epub 2006 Mar 23. Mol Endocrinol. 2006. PMID: 16556734
-
β-Cell replication and islet neogenesis following partial pancreatectomy.Islets. 2011 Jul-Aug;3(4):188-95. doi: 10.4161/isl.3.4.16338. Epub 2011 Jul 1. Islets. 2011. PMID: 21623169
-
Islet morphogenesis and stem cell markers.Cell Biochem Biophys. 2004;40(3 Suppl):81-8. doi: 10.1385/cbb:40:3:81. Cell Biochem Biophys. 2004. PMID: 15289645 Review.
-
Distinctions between islet neogenesis and β-cell replication: implications for reversal of Type 1 and 2 diabetes.J Diabetes. 2010 Jun;2(2):76-84. doi: 10.1111/j.1753-0407.2010.00074.x. Epub 2010 Mar 25. J Diabetes. 2010. PMID: 20923488 Review.
Cited by
-
Islet neogenesis: a possible pathway for beta-cell replenishment.Rev Diabet Stud. 2012 Winter;9(4):407-16. doi: 10.1900/RDS.2012.9.407. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804276 Free PMC article. Review.
-
Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen.Diabetes. 2010 Jan;59(1):143-52. doi: 10.2337/db09-0050. Epub 2009 Oct 15. Diabetes. 2010. PMID: 19833884 Free PMC article.
-
Precursor cells in mouse islets generate new beta-cells in vivo during aging and after islet injury.Endocrinology. 2010 Feb;151(2):520-8. doi: 10.1210/en.2009-0992. Epub 2010 Jan 7. Endocrinology. 2010. PMID: 20056825 Free PMC article.
-
Enhanced Expression of the Key Mitosis Regulator Cyclin B1 Is Mediated by PDZ-Binding Kinase in Islets of Pregnant Mice.J Endocr Soc. 2018 Jan 30;2(3):207-219. doi: 10.1210/js.2017-00338. eCollection 2018 Mar 1. J Endocr Soc. 2018. PMID: 29594255 Free PMC article.
-
EZH2 inhibitors promote β-like cell regeneration in young and adult type 1 diabetes donors.Signal Transduct Target Ther. 2024 Jan 1;9(1):2. doi: 10.1038/s41392-023-01707-x. Signal Transduct Target Ther. 2024. PMID: 38161208 Free PMC article.
References
-
- Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems J-A, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DER, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JRT: International Trial of the Edmonton Protocol for Islet Transplantation. N Engl J Med 355: 1318–1330, 2006 - PubMed
-
- Finegood DT, Scaglia L, Bonner-Weir S: Dynamics of β-cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes 44: 249–256, 1995 - PubMed
-
- Ackermann AM, Gannon M: Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol 38: 193–206, 2007 - PubMed
-
- Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 - PubMed
-
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA: Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12: 817–826, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

