Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial
- PMID: 18728266
- PMCID: PMC2684336
- DOI: 10.1001/jama.300.8.924
Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial
Abstract
Context: Hyperuricemia is a predictor for the development of hypertension and is commonly present in new-onset essential hypertension. Experimentally increasing uric acid levels using a uricase inhibitor causes systemic hypertension in animal models.
Objective: To determine whether lowering uric acid lowers blood pressure (BP) in hyperuricemic adolescents with newly diagnosed hypertension.
Design, setting, and patients: Randomized, double-blind, placebo-controlled, crossover trial (September 2004-March 2007) involving 30 adolescents (aged 11-17 years) who had newly diagnosed, never-treated stage 1 essential hypertension and serum uric acid levels > or = 6 mg/dL. Participants were treated at the Pediatric Hypertension Clinic at Texas Children's Hospital in Houston. Patients were excluded if they had stage 2 hypertension or known renal, cardiovascular, gastrointestinal tract, hepatic, or endocrine disease.
Intervention: Allopurinol, 200 mg twice daily for 4 weeks, and placebo, twice daily for 4 weeks, with a 2-week washout period between treatments. The order of the treatments was randomized.
Main outcome measures: Change in casual and ambulatory blood pressure.
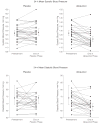
Results: For casual BP, the mean change in systolic BP for allopurinol was -6.9 mm Hg (95% confidence interval [CI], -4.5 to -9.3 mm Hg) vs -2.0 mm Hg (95% CI, 0.3 to -4.3 mm Hg; P = .009) for placebo, and the mean change in diastolic BP for allopurinol was -5.1 mm Hg (95% CI, -2.5 to -7.8 mm Hg) vs -2.4 (95% CI, 0.2 to -4.1; P = .05) for placebo. Mean change in mean 24-hour ambulatory systolic BP for allopurinol was -6.3 mm Hg (95% CI, -3.8 to -8.9 mm Hg) vs 0.8 mm Hg (95% CI, 3.4 to -2.9 mm Hg; P = .001) for placebo and mean 24-hour ambulatory diastolic BP for allopurinol was -4.6 mm Hg (-2.4 to -6.8 mm Hg) vs -0.3 mm Hg (95% CI, 2.3 to -2.1 mm Hg; P = .004) for placebo. Twenty of the 30 participants achieved normal BP by casual and ambulatory criteria while taking allopurinol vs 1 participant while taking placebo (P < .001).
Conclusions: In this short-term, crossover study of adolescents with newly diagnosed hypertension, treatment with allopurinol resulted in reduction of BP. The results represent a new potential therapeutic approach, although not a fully developed therapeutic strategy due to potential adverse effects. These preliminary findings require confirmation in larger clinical trials.
Trial registration: clinicaltrials.gov Identifier: NCT00288184.
Figures
Comment in
-
Allopurinol and the role of uric acid in hypertension.JAMA. 2009 Jan 21;301(3):270; author reply 270-1. doi: 10.1001/jama.2008.1013. JAMA. 2009. PMID: 19155448 No abstract available.
-
[Does treatment with allopurinol reduce blood pressure in adolescents recently diagnosed with essential hypertension?].Nefrologia. 2009;29(6 Suppl):30-2. doi: 10.3265/NEFROLOGIA.2009.29.S.E.noID.36.free. Nefrologia. 2009. PMID: 20221221 Spanish. No abstract available.
References
-
- Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275(9):457–464. - PubMed
-
- Williams J. The total nonprotein nitrogen constituents of the blood in arterial hypertension. Arch Intern Med. 1922;27:748–754.
-
- Mohamed FA. On chronic Bright’s disease, and its essential symptoms. Lancet. 1879;1:399–401.
-
- Davis N. The cardiovascular and renal relations and manifestations of gout. JAMA. 1897;29:267–262.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

