Domoic acid toxicologic pathology: a review
- PMID: 18728725
- PMCID: PMC2525487
- DOI: 10.3390/md20080010
Domoic acid toxicologic pathology: a review
Abstract
Domoic acid was identified as the toxin responsible for an outbreak of human poisoning that occurred in Canada in 1987 following consumption of contaminated blue mussels [Mytilus edulis]. The poisoning was characterized by a constellation of clinical symptoms and signs. Among the most prominent features described was memory impairment which led to the name Amnesic Shellfish Poisoning [ASP]. Domoic acid is produced by certain marine organisms, such as the red alga Chondria armata and planktonic diatom of the genus Pseudo-nitzschia. Since 1987, monitoring programs have been successful in preventing other human incidents of ASP. However, there are documented cases of domoic acid intoxication in wild animals and outbreaks of coastal water contamination in many regions world-wide. Hence domoic acid continues to pose a global risk to the health and safety of humans and wildlife. Several mechanisms have been implicated as mediators for the effects of domoic acid. Of particular importance is the role played by glutamate receptors as mediators of excitatory neurotransmission and the demonstration of a wide distribution of these receptors outside the central nervous system, prompting the attention to other tissues as potential target sites. The aim of this document is to provide a comprehensive review of ASP, DOM induced pathology including ultrastructural changes associated to subchronic oral exposure, and discussion of key proposed mechanisms of cell/tissue injury involved in DOM induced brain pathology and considerations relevant to food safety and human health.
Keywords: Amnesic Shellfish Poisoning; Domoic Acid; Excitotoxicity; Food Safety; Glutamate Receptors; Neuropathology; Neurotoxicology; Toxicologic Pathology.
Figures
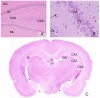




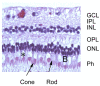



References
-
- Hynie I, Hockin J, Wright J, Iverson F. Panel discussion: evidence that domoic acid was the cause of the 1987 outbreak. Can Dis Wkly Rep. 1990;16(Suppl 1E):37–40. - PubMed
-
- Todd EC. Chronology of the toxic mussels outbreak. Can Dis Wkly Rep. 1990;16(Suppl 1E):3–4. - PubMed
-
- Liston AJ. Domoic acid toxicity. Introduction. Can Dis Wkly Rep. 1990;16(Suppl 1E):1–2. - PubMed
-
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990;322(25):1775–1780. - PubMed
-
- Quilliam MA, Wright JL. The amnesic shellfish poisoning mystery. Anal Chem. 1989;61(18):1053A–1106A. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
