Adenine nucleotide translocator transports haem precursors into mitochondria
- PMID: 18728780
- PMCID: PMC2516936
- DOI: 10.1371/journal.pone.0003070
Adenine nucleotide translocator transports haem precursors into mitochondria
Abstract
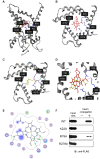
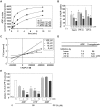
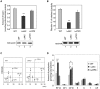
Haem is a prosthetic group for haem proteins, which play an essential role in oxygen transport, respiration, signal transduction, and detoxification. In haem biosynthesis, the haem precursor protoporphyrin IX (PP IX) must be accumulated into the mitochondrial matrix across the inner membrane, but its mechanism is largely unclear. Here we show that adenine nucleotide translocator (ANT), the inner membrane transporter, contributes to haem biosynthesis by facilitating mitochondrial accumulation of its precursors. We identified that haem and PP IX specifically bind to ANT. Mitochondrial uptake of PP IX was inhibited by ADP, a known substrate of ANT. Conversely, ADP uptake into mitochondria was competitively inhibited by haem and its precursors, suggesting that haem-related porphyrins are accumulated into mitochondria via ANT. Furthermore, disruption of the ANT genes in yeast resulted in a reduction of haem biosynthesis by blocking the translocation of haem precursors into the matrix. Our results represent a new model that ANT plays a crucial role in haem biosynthesis by facilitating accumulation of its precursors into the mitochondrial matrix.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

