Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties
- PMID: 18728788
- PMCID: PMC2518121
- DOI: 10.1371/journal.pone.0003077
Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties
Abstract
Background: Cancer stem cells (CSCs) are thought to be responsible for tumor regeneration after chemotherapy, although direct confirmation of this remains forthcoming. We therefore investigated whether drug treatment could enrich and maintain CSCs and whether the high tumorogenic and metastatic abilities of CSCs were based on their marked ability to produce growth and angiogenic factors and express their cognate receptors to stimulate tumor cell proliferation and stroma formation.
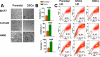
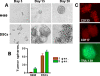
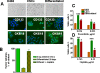
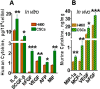
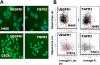
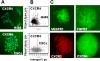
Methodology/findings: Treatment of lung tumor cells with doxorubicin, cisplatin, or etoposide resulted in the selection of drug surviving cells (DSCs). These cells expressed CD133, CD117, SSEA-3, TRA1-81, Oct-4, and nuclear beta-catenin and lost expression of the differentiation markers cytokeratins 8/18 (CK 8/18). DSCs were able to grow as tumor spheres, maintain self-renewal capacity, and differentiate. Differentiated progenitors lost expression of CD133, gained CK 8/18 and acquired drug sensitivity. In the presence of drugs, differentiation of DSCs was abrogated allowing propagation of cells with CSC-like characteristics. Lung DSCs demonstrated high tumorogenic and metastatic potential following inoculation into SCID mice, which supported their classification as CSCs. Luminex analysis of human and murine cytokines in sonicated lysates of parental- and CSC-derived tumors revealed that CSC-derived tumors contained two- to three-fold higher levels of human angiogenic and growth factors (VEGF, bFGF, IL-6, IL-8, HGF, PDGF-BB, G-CSF, and SCGF-beta). CSCs also showed elevated levels of expression of human VEGFR2, FGFR2, CXCR1, 2 and 4 receptors. Moreover, human CSCs growing in SCID mice stimulated murine stroma to produce elevated levels of angiogenic and growth factors.
Conclusions/significance: These findings suggest that chemotherapy can lead to propagation of CSCs and prevention of their differentiation. The high tumorigenic and metastatic potentials of CSCs are associated with efficient cytokine network production that may represent a target for increased efficacy of cancer therapy.
Conflict of interest statement
Figures




References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
-
- Collins AT, Maitland NJ. Prostate cancer stem cells. Eur J Cancer. 2006;42:1213–1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

