A review of ramelteon in the treatment of sleep disorders
- PMID: 18728808
- PMCID: PMC2515902
- DOI: 10.2147/ndt.s483
A review of ramelteon in the treatment of sleep disorders
Abstract
Ramelteon is a selective melatonin receptor (MT(1) and MT(2)) agonist that has been approved by the US Food and Drug Administration for the treatment of insomnia characterized by difficulty with sleep onset. It is the only approved sleep-promoting medication that does not have a direct sedating effect, but rather enhances sleep through effects on sleep regulatory mechanisms within the suprachiasmatic nucleus. Ramelteon has been shown to have no abuse liability and therefore is not scheduled by the U.S. Drug Enforcement Agency as a controlled substance. It is available as an 8 mg tablet, which should be taken approximately 30 minutes prior to bedtime. The FDA approval contains no limitation on how long the medication may be prescribed.
Keywords: circadian rhythm; insomnia; melatonin receptors; ramelteon; sleep-wake cycle.
Figures
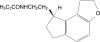

References
-
- Amakye DD, Hibberd M, Stevenson SJ. A phase I study to investigate the absolute bioavailability of a single oral dose of ramelteon (TAK-375) in healthy male subjects. Sleep. 2004;27(Abstract Suppl):A54.
-
- DeMicco M, Wang-Weigand S, Zhang J. Long-term therapeutic effects of ramelteon treatment in adults with chronic insomnia: A 1-year study. Sleep. 2006;29(Abstract Suppl):A234.
-
- Erman M, Seiden D, Zammit G, et al. An efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. - PubMed
-
- France CP, Weltman RH, Koek W, et al. Acute and chronic effects of ramelteon in rhesus monkeys (macaca mulatta): dependence liability studies. Behav Neurosci. 2006;120:535–41. - PubMed
-
- Greenblatt DJ, Harmatz JS, Karim A. Age and gender effects on the pharmacokinetics and pharmacodynamics of ramelteon, a hypnotic agent acting via melatonin receptors MT1 and MT2. J Clin Pharmacol. 2007;47:485–96. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

