Optimizing levodopa therapy for Parkinson's disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective
- PMID: 18728816
- PMCID: PMC2515910
- DOI: 10.2147/ndt.s1660
Optimizing levodopa therapy for Parkinson's disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective
Abstract
After 40 years of clinical experience, levodopa remains the gold standard treatment for Parkinson's disease (PD) despite the recent emergence of a host of new therapies. Some physicians are cautious when prescribing levodopa because of its association with motor complications. Evidence now suggests that levodopa-associated complications are a result of deep troughs in delivery of levodopa to the brain caused by the short plasma half-life of conventional levodopa formulations (levodopa and a dopa decarboxylase inhibitor [DDCI]). Dosing strategies, such as dose increases and dose fractionation, may be effective in the short term. For the longer-term, levodopa/carbidopa/entacapone provides pharmacokinetically optimized levodopa therapy that significantly increases the plasma half-life and bioavailability of levodopa, providing more consistent plasma levodopa levels without deep troughs. Evidence from clinical trials in PD patients experiencing re-emergence of symptoms due to wearing-off has consistently shown that levodopa/DDCI and entacapone significantly increases ON-time and affords greater functionality, as measured by the Unified Parkinson's Disease Rating Scale (UPDRS) with conventional levodopa. These trials have also shown that levodopa/DDCI and entacapone is generally well tolerated, with notable adverse events including worsening dyskinesia, nausea and diarrhea. Patients experiencing re-emergence of symptoms due to wearing-off may benefit from optimized levodopa therapy with levodopa/carbidopa/entacapone.
Keywords: Stalevo; dyskinesia; entacapone; levodopa; wearing-off.
Figures

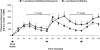
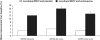
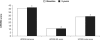
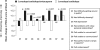
References
-
- Chapuis S, et al. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov Disord. 2005;20:224–30. - PubMed
-
- Chaudhuri KR, et al. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–45. - PubMed
-
- de la Fuente-Fernandez R, et al. Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol. 2001;49:298–303. - PubMed
-
- Dorsey ER, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–6. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

