Effects of vanadium-containing compounds on membrane lipids and on microdomains used in receptor-mediated signaling
- PMID: 18729092
- PMCID: PMC3159192
- DOI: 10.1002/cbdv.200890144
Effects of vanadium-containing compounds on membrane lipids and on microdomains used in receptor-mediated signaling
Abstract
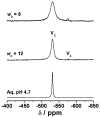
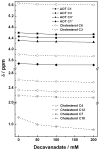
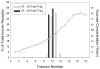
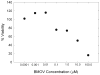
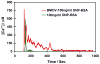
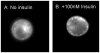
There is increasing evidence for the involvement of plasma membrane microdomains in insulin receptor function. Moreover, disruption of these structures, which are typically enriched in sphingomyelin and cholesterol, results in insulin resistance. Treatment strategies for insulin resistance include the use of vanadium (V) compounds which have been shown in animal models to enhance insulin responsiveness. One possible mechanism for insulin-enhancing effects might involve direct effects of V compounds on membrane lipid organization. These changes in lipid organization promote the partitioning of insulin receptors and other receptors into membrane microdomains where receptors are optimally functional. To explore this possibility, we have used several strategies involving V complexes such as [VO(2)(dipic)](-) (pyridin-2,6-dicarboxylatodioxovanadium(V)), decavanadate (V(10)O(28)(6-), V(10)), BMOV (bis(maltolato)oxovanadium(IV)), and [VO(saltris)](2) (2-salicylideniminato-2-(hydroxymethyl)-1,3-dihydroxypropane-oxovanadium(V)). Our strategies include an evaluation of interactions between V-containing compounds and model lipid systems, an evaluation of the effects of V compounds on lipid fluidity in erythrocyte membranes, and studies of the effects of V-containing compounds on signaling events initiated by receptors known to use membrane microdomains as signaling platforms.
Figures
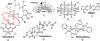

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

