Detection and characterization of altered conformations of protein pharmaceuticals using complementary mass spectrometry-based approaches
- PMID: 18729476
- PMCID: PMC2628293
- DOI: 10.1021/ac801214x
Detection and characterization of altered conformations of protein pharmaceuticals using complementary mass spectrometry-based approaches
Abstract
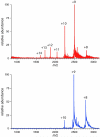
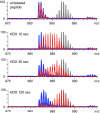
Unlike small-molecule drugs, the conformational properties of protein biopharmaceuticals in solution are influenced by a variety of factors that are not solely defined by their covalent chemical structure. Since the conformation (or higher order structure) of a protein is a major modulator of its biological activity, the ability to detect changes in both the higher order structure and conformational dynamics of a protein, induced by an array of extrinsic factors, is of central importance in producing, purifying, and formulating a commercial biopharmaceutical with consistent therapeutic properties. In this study we demonstrate that two complementary mass spectrometry-based approaches (analysis of ionic charge-state distribution and hydrogen/deuterium exchange) can be a potent tool in monitoring conformational changes in protein biopharmaceuticals. The utility of these approaches is demonstrated by detecting and characterizing conformational changes in the biopharmaceutical product interferon beta-1a (IFN-beta-1a). The protein degradation process was modeled by inducing a single chemical modification of IFN-beta1a (alkylation of its only free cysteine residue with N-ethylmaleimide), which causes significant reduction in its antiviral activity. Analysis of IFN-beta1a ionic charge-state distributions unequivocally reveals a significant decrease of conformational stability in the degraded protein, while hydrogen/deuterium exchange measurements provide a clear indication that the higher order structure is affected well beyond the covalent modification site. Importantly, neither technique required that the location or indeed the nature of the chemical modification be known prior to or elucidated in the process of the analysis. In contrast, application of the standard armamentarium of biophysical tools, which are commonly employed for quality control of protein pharmaceuticals, met with very limited success in detection and characterization of conformational changes in the modified IFN-beta1a. This work highlights the role mass spectrometry can and should play in the biopharmaceutical industry beyond the presently assigned task of primary structure analysis.
Figures




References
-
- Walsh G. Biopharmaceutical benchmarks 2006. Nat. Biotech. 2006;24:769–776. - PubMed
-
- Lawrence S. Pipelines turn to biotech. Nat. Biotech. 2007;25:1342–1342. - PubMed
-
- Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv. Drug Del. Rev. 2004;56:335–347. - PubMed
-
- Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, Morris J. Ritonavir: an extraordinary example of conformational polymorphism. Pharm. Res. 2001;18:859–866. - PubMed
-
- Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MFBG. A role for protein misfolding in immunogenicity of biopharmaceuticals. J. Biol. Chem. 2007;282:2229–2236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

