Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis
- PMID: 18752325
- PMCID: PMC2929828
- DOI: 10.1002/hep.22401
Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis
Erratum in
- Hepatology. 2009 Jul;50(1):331
Abstract
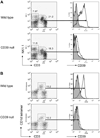
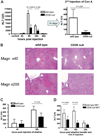
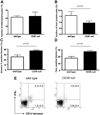
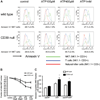
Concanavalin A (Con A)-induced injury is an established natural killer T (NKT) cell-mediated model of inflammation that has been used in studies of immune liver disease. Extracellular nucleotides, such as adenosine triphosphate, are released by Con A-stimulated cells and bind to specific purinergic type 2 receptors to modulate immune activation responses. Levels of extracellular nucleotides are in turn closely regulated by ectonucleotidases, such as CD39/NTPDase1. Effects of extracellular nucleotides and CD39 on NKT cell activation and upon hepatic inflammation have been largely unexplored to date. Here, we show that NKT cells express both CD39 and CD73/ecto-5'-nucleotidase and can therefore generate adenosine from extracellular nucleotides, whereas natural killer cells do not express CD73. In vivo, mice null for CD39 are protected from Con A-induced liver injury and show substantively lower serum levels of interleukin-4 and interferon-gamma when compared with matched wild-type mice. Numbers of hepatic NKT cells are significantly decreased in CD39 null mice after Con A administration. Hepatic NKT cells express most P2X and P2Y receptors; exceptions include P2X3 and P2Y11. Heightened levels of apoptosis of CD39 null NKT cells in vivo and in vitro appear to be driven by unimpeded activation of the P2X7 receptor.
Conclusion: CD39 and CD73 are novel phenotypic markers of NKT cells. In turn, CD39 expression [corrected] modulates nucleotide-mediated cytokine production by, and limits apoptosis of, hepatic NKT cells. Deletion of CD39 is protective in [corrected] Con A-induced hepatitis. This study illustrates a [corrected] role for purinergic signaling in NKT-mediated mechanisms that result in liver immune injury.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures




References
-
- Exley MA, Koziel MJ. To be or not to be NKT: natural killer T cells in the liver. HEPATOLOGY. 2004;40:1033–1040. - PubMed
-
- Toyabe S, Seki S, Iiai T, Takeda K, Shirai K, Watanabe H, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–1542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
