Texture coding in the rat whisker system: slip-stick versus differential resonance
- PMID: 18752354
- PMCID: PMC2525689
- DOI: 10.1371/journal.pbio.0060215
Texture coding in the rat whisker system: slip-stick versus differential resonance
Abstract
Rats discriminate surface textures using their whiskers (vibrissae), but how whiskers extract texture information, and how this information is encoded by the brain, are not known. In the resonance model, whisker motion across different textures excites mechanical resonance in distinct subsets of whiskers, due to variation across whiskers in resonance frequency, which varies with whisker length. Texture information is therefore encoded by the spatial pattern of activated whiskers. In the competing kinetic signature model, different textures excite resonance equally across whiskers, and instead, texture is encoded by characteristic, nonuniform temporal patterns of whisker motion. We tested these models by measuring whisker motion in awake, behaving rats whisking in air and onto sandpaper surfaces. Resonant motion was prominent during whisking in air, with fundamental frequencies ranging from approximately 35 Hz for the long Delta whisker to approximately 110 Hz for the shorter D3 whisker. Resonant vibrations also occurred while whisking against textures, but the amplitude of resonance within single whiskers was independent of texture, contradicting the resonance model. Rather, whiskers resonated transiently during discrete, high-velocity, and high-acceleration slip-stick events, which occurred prominently during whisking on surfaces. The rate and magnitude of slip-stick events varied systematically with texture. These results suggest that texture is encoded not by differential resonant motion across whiskers, but by the magnitude and temporal pattern of slip-stick motion. These findings predict a temporal code for texture in neural spike trains.
Conflict of interest statement
Figures
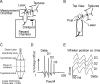

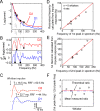





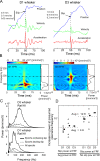


Comment in
-
Whisker-mediated texture discrimination.PLoS Biol. 2008 Aug 26;6(8):e220. doi: 10.1371/journal.pbio.0060220. PLoS Biol. 2008. PMID: 18752356 Free PMC article.
References
-
- Heller MA. Texture perception in sighted and blind observers. Percept Psychophys. 1989;45:49–54. - PubMed
-
- Prigg T, Goldreich D, Carvell GE, Simons DJ. Texture discrimination and unit recordings in the rat whisker/barrel system. Physiol Behav. 2002;77:671–675. - PubMed
-
- Guic-Robles E, Valdivieso C, Guajardo G. Rats can learn a roughness discrimination using only their vibrissal system. Behav Brain Res. 1989;31:285–289. - PubMed
-
- Guic-Robles E, Jenkins WM, Bravo H. Vibrissal roughness discrimination is barrelcortex-dependent. Behav Brain Res. 1992;48:145–152. - PubMed

