Stable high volumetric production of glycosylated human recombinant IFNalpha2b in HEK293 cells
- PMID: 18752669
- PMCID: PMC2538527
- DOI: 10.1186/1472-6750-8-65
Stable high volumetric production of glycosylated human recombinant IFNalpha2b in HEK293 cells
Abstract
Background: Mammalian cells are becoming the prevailing expression system for the production of recombinant proteins because of their capacity for proper protein folding, assembly, and post-translational modifications. These systems currently allow high volumetric production of monoclonal recombinant antibodies in the range of grams per litre. However their use for large-scale expression of cytokines typically results in much lower volumetric productivity.
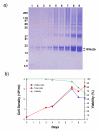
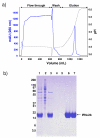
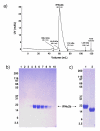
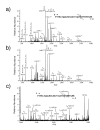
Results: We have engineered a HEK293 cell clone for high level production of human recombinant glycosylated IFNalpha2b and developed a rapid and efficient method for its purification. This clone steadily produces more than 200 mg (up to 333 mg) of human recombinant IFNalpha2b per liter of serum-free culture, which can be purified by a single-step cation-exchange chromatography following media acidification and clarification. This rapid procedure yields 98% pure IFNalpha2b with a recovery greater than 70%. Purified IFNalpha2b migrates on SDS-PAGE as two species, a major 21 kDa band and a minor 19 kDa band. N-terminal sequences of both forms are identical and correspond to the expected mature protein. Purified IFNalpha2b elutes at neutral pH as a single peak with an apparent molecular weight of 44,000 Da as determined by size-exclusion chromatography. The presence of intramolecular and absence of intermolecular disulfide bridges is evidenced by the fact that non-reduced IFNalpha2b has a greater electrophoretic mobility than the reduced form. Treatment of purified IFNalpha2b with neuraminidase followed by O-glycosidase both increases electrophoretic mobility, indicating the presence of sialylated O-linked glycan. A detailed analysis of glycosylation by mass spectroscopy identifies disialylated and monosialylated forms as the major constituents of purified IFNalpha2b. Electron transfer dissociation (ETD) shows that the glycans are linked to the expected threonine at position 106. Other minor glycosylated forms and non-sialylated species are also detected, similar to IFNalpha2b produced naturally by lymphocytes. Further, the HEK293-produced IFNalpha2b is biologically active as shown with reporter gene and antiviral assays.
Conclusion: These results show that the HEK293 cell line is an efficient and valuable host for the production of biologically active and glycosylated human IFNalpha2b.
Figures




References
-
- Kaluz S, Kabat P, Gibadulinova A, Vojtassak J, Fuchsberger N, Kontsek P. Interferon alpha2b is the predominant subvariant detected in human genomic DNAs. Acta Virol. 1994;38:101–104. - PubMed
-
- Gewert DR, Sharp NA, Barber KA, Cooper H, Tucker D, Lewis AP, Thursz M, Crowe JS. Detection of rare allelic variants of the interferon-alpha 2 gene in human genomic DNA. J Interferon Cytokine Res. 1995;15:403–406. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

