The co-administration of quetiapine or placebo to cognitive-behavior therapy in treatment refractory depression: a preliminary trial
- PMID: 18752690
- PMCID: PMC2553785
- DOI: 10.1186/1471-244X-8-73
The co-administration of quetiapine or placebo to cognitive-behavior therapy in treatment refractory depression: a preliminary trial
Abstract
Background: Patients with major depression refractory to repeated pharmacological trials (TRD) may remain symptomatic for many years after their index episode. Augmentation strategies (with lithium or an atypical antipsychotic) or combining an antidepressant with short-term psychotherapy have been used with relative success in these patients. The aim of this study was to assess the effectiveness of the concomitant administration of quetiapine, an atypical antipsychotic, or placebo, to cognitive-behavior therapy (CBT) in TRD.
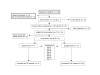
Methods: Thirty-one patients who met entrance criteria for unipolar major depression (TRD stage II or greater) underwent 3 weeks of lithium augmentation after which non-responders (N = 22) were randomized to receive either quetiapine or placebo as an adjunct to their 12 weekly CBT sessions (quetiapine/CBT or placebo/CBT groups). Primary efficacy measures were the Hamilton and the Montgomery-Asberg rating scales for depression.
Results: Overall, there was a significant reduction in both primary efficacy measure scores at LOCF for the 11 patients in the quetiapine/CBT group but not in the placebo/CBT treated patients. Patients in the quetiapine/CBT group, compared to those receiving placebo/CBT, showed a significantly greater degree of improvement on one primary and one secondary efficacy measure, were more likely to complete the trial and, completed a greater number of CBT sessions.
Conclusion: Although preliminary, our results suggest that the adjunctive administration of quetiapine to CBT may prove useful in the treatment of stage II TRD.
Trial registration: Current Controlled Trials ISRCTN12638696.
References
-
- Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52:46–54. - PubMed
-
- Bauer M, Adli M, Baethge C, Berghöfer A, Sasse J, Heinz A, Bschor T. Lithium augmentation in refractory depression: clinical evidence and neurobiological mechanisms. Can J Psychiatry. 2003;48:440–8. - PubMed
-
- deMontigny C, Chaput Y, Blier P. Lithium augmentation of antidepressant treatments: evidence for the involvement of the 5-HT system? In: Briley M, Filion G, editor. New Concepts in depression. London MacMillan Press; 1988. pp. 144–160.
-
- Marangell LB. Swtiching antidepressants for treatment-resistant major depression. J Clin Psychiatry. 2001;62 Suppl 18:12–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous