Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions
- PMID: 18753129
- PMCID: PMC2581552
- DOI: 10.1074/jbc.M804784200
Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions
Abstract
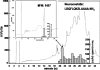
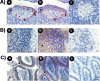
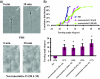
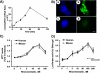
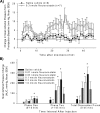
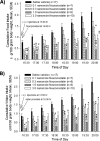
Somatostatin is important in the regulation of diverse neuroendocrine functions. Based on bioinformatic analyses of evolutionarily conserved sequences, we predicted another peptide hormone in pro-somatostatin and named it neuronostatin. Immuno-affinity purification allowed the sequencing of an amidated neuronostatin peptide of 13 residues from porcine tissues. In vivo treatment with neuronostatin induced c-Fos expression in gastrointestinal tissues, anterior pituitary, cerebellum, and hippocampus. In vitro treatment with neuronostatin promoted the migration of cerebellar granule cells and elicited direct depolarizing actions on paraventricular neurons in hypothalamic slices. In a gastric tumor cell line, neuronostatin induced c-Fos expression, stimulated SRE reporter activity, and promoted cell proliferation. Furthermore, intracerebroventricular treatment with neuronostatin increased blood pressure but suppressed food intake and water drinking. Our findings demonstrate diverse neuronal, neuroendocrine, and cardiovascular actions of a somatostatin gene-encoded hormone and provide the basis to investigate the physiological roles of this endogenously produced brain/gut peptide.
Figures



References
-
- Vale, W., Brazeau, P., Grant, G., Nussey, A., Burgus, R., Rivier, J., Ling, N., and Guillemin, R. (1972) C. R. Acad. Sci. Hebd. Seances. Acad. Sci. D 275 2913-2916 - PubMed
-
- Brazeau, P., Vale, W., Burgus, R., Ling, N., Butcher, M., Rivier, J., and Guillemin, R. (1973) Science 179 77-79 - PubMed
-
- Reichlin, S. (1983) N. Engl. J. Med. 309 1495-1501 - PubMed
-
- Lamberts, S. W., Krenning, E. P., and Reubi, J. C. (1991) Endocr. Rev. 12 450-482 - PubMed
-
- Low, M. J. (2004) Best Pract. Res. Clin. Endocrinol. Metab. 18 607-622 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

