DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility
- PMID: 18753132
- PMCID: PMC2576531
- DOI: 10.1074/jbc.M806085200
DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility
Abstract
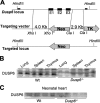
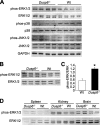
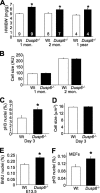
The strength and duration of mitogen-activated protein kinase signaling is regulated through phosphorylation and dephosphorylation by dedicated dual-specificity kinases and phosphatases, respectively. Here we investigated the physiological role that extracellular signal-regulated kinases 1/2 (ERK1/2) dephosphorylation plays in vivo through targeted disruption of the gene encoding dual-specificity phosphatase 6 (Dusp6) in the mouse. Dusp6(-/-) mice, which were viable, fertile, and otherwise overtly normal, showed an increase in basal ERK1/2 phosphorylation in the heart, spleen, kidney, brain, and fibroblasts, but no change in ERK5, p38, or c-Jun N-terminal kinases activation. However, loss of Dusp6 did not increase or prolong ERK1/2 activation after stimulation, suggesting that its function is more dedicated to basal ERK1/2 signaling tone. In-depth analysis of the physiological effect associated with increased baseline ERK1/2 signaling was performed in cultured mouse embryonic fibroblasts (MEFs) and the heart. Interestingly, mice lacking Dusp6 had larger hearts at every age examined, which was associated with greater rates of myocyte proliferation during embryonic development and in the early postnatal period, resulting in cardiac hypercellularity. This increase in myocyte content in the heart was protective against decompensation and hypertrophic cardiomyopathy following long term pressure overload and myocardial infarction injury in adult mice. Dusp6(-/-) MEFs also showed reduced apoptosis rates compared with wild-type MEFs. These results demonstrate that ERK1/2 signaling is physiologically restrained by DUSP6 in coordinating cellular development and survival characteristics, directly impacting disease-responsiveness in adulthood.
Figures




References
-
- Garrington, T. P., and Johnson, G. L. (1999) Curr. Opin. Cell Biol. 11 211-218 - PubMed
-
- Alvarado-Kristensson, M., and Andersson, T. (2005) J. Biol. Chem. 280 6238-6244 - PubMed
-
- Liu, Q., and Hofmann, P. A. (2004) Am. J. Physiol. 286 H2204-H2212 - PubMed
-
- Sundaresan, P., and Farndale, R. W. (2002) FEBS Lett. 528 139-144 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

