Expression of S100A6 in cardiac myocytes limits apoptosis induced by tumor necrosis factor-alpha
- PMID: 18753141
- PMCID: PMC2662078
- DOI: 10.1074/jbc.M805318200
Expression of S100A6 in cardiac myocytes limits apoptosis induced by tumor necrosis factor-alpha
Abstract
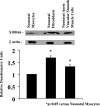
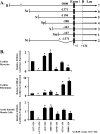
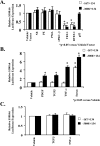
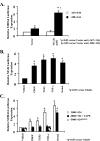
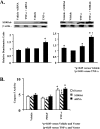
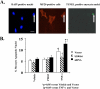
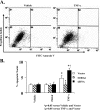
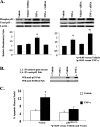
S100A6 is induced in myocardium post-infarction in vivo and in response to growth factors and inflammatory cytokines in vitro. Forced expression of S100A6 in cardiomyocytes inhibits regulation of cardiac specific gene expression in response to trophic stimulation. To define regulation and function of S100A6, we characterized the human S100A6 promoter and mapped upstream regulatory elements in rat neonatal cardiac myocytes, fibroblasts, and vascular smooth muscle cells and defined a functional role for S100A6 in tumor necrosis factor-alpha-induced myocyte apoptosis. The functional S100A6 promoter was localized to region -167/+134 containing 167 upstream base pairs. The S100A6 promoter is regulated by positive (-361/-167 and -588/-361) and negative (-1371/-1194) elements. Tumor necrosis factor-alpha induced the maximal S100A6 promoter and transcription factor NF-kappaB (p65 subunit). Electrophoretic mobility shift showed that tumor necrosis factor-alpha induced p65 binding to a potential NF-kappaB-binding site at -460/-451. Chromatin immunoprecipitation analysis revealed p65 is recruited to the S100A6 promoter upon tumor necrosis factor-alpha stimulation. The NF-kappaB inhibitor caffeic acid phenethyl ester and mutation of the NF-kappaB-binding site inhibited S100A6 promoter activation by tumor necrosis factor-alpha. Tumor necrosis factor-alpha induced cardiac myocyte apoptosis. Specific inhibition of S100A6 using a small interfering RNA directed against S100A6 potentiated tumor necrosis factor-alpha-induced myocyte apoptosis, whereas overexpression of S100A6 by gene transfer prevented tumor necrosis factor-alpha-induced myocyte apoptosis by interfering with p53 phosphorylation. These results demonstrate that S100A6 is induced by tumor necrosis factor-alpha via an NF-kappaB-dependent mechanism, serving a role in homeostasis to limit tumor necrosis factor-alpha-induced apoptosis by regulating p53 phosphorylation.
Figures

References
-
- Kligman, D., and Hilt, D. C. (1988) Trends Biochem. Sci. 13 437-443 - PubMed
-
- Zimmer, D. B., Cornwall, E. H., Landar, A., and Song, W. (1995) Brain Res. Bull. 37 417-429 - PubMed
-
- Schafer, B. W., and Heizmann, C. W. (1996) Trends Biochem. Sci. 21 134-140 - PubMed
-
- Kuznicki, J., Filipek, A., Heimann, P., Kaczmarek, L., and Kaminska, B. (1989) FEBS Lett. 254 141-144 - PubMed
-
- Engelkamp, D., Schafer, B. W., Erne, P., and Heizmann, C. W. (1992) Biochemistry 31 10258-10264 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

