Establishment of murine cytomegalovirus latency in vivo is associated with changes in histone modifications and recruitment of transcriptional repressors to the major immediate-early promoter
- PMID: 18753203
- PMCID: PMC2573207
- DOI: 10.1128/JVI.00865-08
Establishment of murine cytomegalovirus latency in vivo is associated with changes in histone modifications and recruitment of transcriptional repressors to the major immediate-early promoter
Abstract
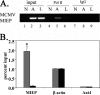
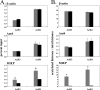
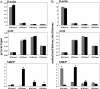
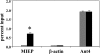
Human cytomegalovirus (CMV) is a ubiquitous herpesvirus with the ability to establish a lifelong latent infection. The mechanism by which this occurs is not well understood. Regulation of, for example, immediate-early (IE) gene expression is thought to be a critical control point in transcriptional control of the switch between latency and reactivation. Here, we present evidence that supports previous studies showing that the majority of genomes are quiescent with respect to gene expression. To study the possible role of epigenetic factors that may be involved in repression of ie gene expression in latency, we have analyzed changes in the patterns of modifications of histones bound to the major IE promoter (MIEP) in the kidneys of acutely and latently infected mice. Our studies show that, like herpes simplex virus, murine CMV genomes become relatively enriched in histones in latent infection. There are dramatic changes in modifications of histones associated with the MIEP when latency is established: H3 and H4 become hypoacetylated and H3 is hypomethylated at lysine 4, while H3 lysine 9 is hypermethylated in latently infected mice. These changes are accompanied by a relative loss of RNA polymerase and gain of heterochromatin protein 1gamma and Yin-Yang 1 bound to the MIEP. Our studies suggest that, in the majority of cells, CMV establishes a true latent infection, defined as the lack of expression of genes associated with productive infection, and that this occurs through changes in histone modifications and recruitment of transcriptional silencing factors to the MIEP.
Figures




References
-
- Amelio, A. L., N. V. Giordani, N. J. Kubat, E. J. O'Neil, and D. C. Bloom. 2006. Deacetylation of the herpes simplex virus type 1 latency-associated transcript (LAT) enhancer and a decrease in LAT abundance precede an increase in ICP0 transcriptional permissiveness at early times postexplant. J. Virol. 802063-2068. - PMC - PubMed
-
- Bain, M., M. Reeves, and J. Sinclair. 2006. Regulation of human cytomegalovirus gene expression by chromatin remodeling, p. 167-183. In M. Reddehase (ed.), Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Norfolk, United Kingdom.
-
- Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410120-124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

