Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons
- PMID: 18753205
- PMCID: PMC2573198
- DOI: 10.1128/JVI.01241-08
Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons
Abstract
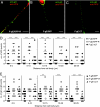
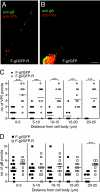
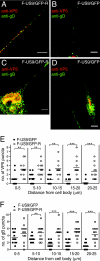
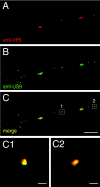
Following reactivation from latency, alphaherpesviruses replicate in sensory neurons and assemble capsids that are transported in the anterograde direction toward axon termini for spread to epithelial tissues. Two models currently describe this transport. The Separate model suggests that capsids are transported in axons independently from viral envelope glycoproteins. The Married model holds that fully assembled enveloped virions are transported in axons. The herpes simplex virus (HSV) membrane glycoprotein heterodimer gE/gI and the US9 protein are important for virus anterograde spread in the nervous systems of animal models. It was not clear whether gE/gI and US9 contribute to the axonal transport of HSV capsids, the transport of membrane proteins, or both. Here, we report that the efficient axonal transport of HSV requires both gE/gI and US9. The transport of both capsids and glycoproteins was dramatically reduced, especially in more distal regions of axons, with gE(-), gI(-), and US9-null mutants. An HSV mutant lacking just the gE cytoplasmic (CT) domain displayed an intermediate reduction in capsid and glycoprotein transport. We concluded that HSV gE/gI and US9 promote the separate transport of both capsids and glycoproteins. gE/gI was transported in association with other HSV glycoproteins, gB and gD, but not with capsids. In contrast, US9 colocalized with capsids and not with membrane glycoproteins. Our observations suggest that gE/gI and US9 function in the neuron cell body to promote the loading of capsids and glycoprotein-containing vesicles onto microtubule motors that ferry HSV structural components toward axon tips.
Figures





Similar articles
-
Herpes Simplex Virus gE/gI and US9 Promote both Envelopment and Sorting of Virus Particles in the Cytoplasm of Neurons, Two Processes That Precede Anterograde Transport in Axons.J Virol. 2017 May 12;91(11):e00050-17. doi: 10.1128/JVI.00050-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331094 Free PMC article.
-
Herpes simplex virus membrane proteins gE/gI and US9 act cooperatively to promote transport of capsids and glycoproteins from neuron cell bodies into initial axon segments.J Virol. 2013 Jan;87(1):403-14. doi: 10.1128/JVI.02465-12. Epub 2012 Oct 17. J Virol. 2013. PMID: 23077321 Free PMC article.
-
Characterization of the Herpes Simplex Virus (HSV) Tegument Proteins That Bind to gE/gI and US9, Which Promote Assembly of HSV and Transport into Neuronal Axons.J Virol. 2020 Nov 9;94(23):e01113-20. doi: 10.1128/JVI.01113-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32938770 Free PMC article.
-
Anterograde transport of α-herpesviruses in neuronal axons.Virology. 2021 Jul;559:65-73. doi: 10.1016/j.virol.2021.02.011. Epub 2021 Mar 4. Virology. 2021. PMID: 33836340 Review.
-
Transport and egress of herpes simplex virus in neurons.Rev Med Virol. 2008 Jan-Feb;18(1):35-51. doi: 10.1002/rmv.560. Rev Med Virol. 2008. PMID: 17992661 Review.
Cited by
-
Molecular association of herpes simplex virus type 1 glycoprotein E with membrane protein Us9.Arch Virol. 2016 Nov;161(11):3203-13. doi: 10.1007/s00705-016-3028-z. Epub 2016 Aug 27. Arch Virol. 2016. PMID: 27568015 Free PMC article.
-
Infection and Transport of Herpes Simplex Virus Type 1 in Neurons: Role of the Cytoskeleton.Viruses. 2018 Feb 23;10(2):92. doi: 10.3390/v10020092. Viruses. 2018. PMID: 29473915 Free PMC article. Review.
-
Motor Skills: Recruitment of Kinesins, Myosins and Dynein during Assembly and Egress of Alphaherpesviruses.Viruses. 2021 Aug 17;13(8):1622. doi: 10.3390/v13081622. Viruses. 2021. PMID: 34452486 Free PMC article. Review.
-
Anterograde transport of herpes simplex virus capsids in neurons by both separate and married mechanisms.J Virol. 2011 Jun;85(12):5919-28. doi: 10.1128/JVI.00116-11. Epub 2011 Mar 30. J Virol. 2011. PMID: 21450818 Free PMC article.
-
Deletion of the us7 and us8 genes of pseudorabies virus exerts a differential effect on the expression of early and late viral genes.Virus Genes. 2017 Aug;53(4):603-612. doi: 10.1007/s11262-017-1465-8. Epub 2017 May 5. Virus Genes. 2017. PMID: 28477233
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

