Bluetongue virus outer capsid protein VP5 interacts with membrane lipid rafts via a SNARE domain
- PMID: 18753209
- PMCID: PMC2573168
- DOI: 10.1128/JVI.01274-08
Bluetongue virus outer capsid protein VP5 interacts with membrane lipid rafts via a SNARE domain
Abstract
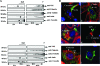
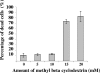
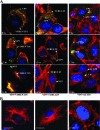
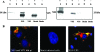
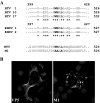
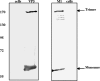
Bluetongue virus (BTV) is a nonenveloped double-stranded RNA virus belonging to the family Reoviridae. The two outer capsid proteins, VP2 and VP5, are responsible for virus entry. However, little is known about the roles of these two proteins, particularly VP5, in virus trafficking and assembly. In this study, we used density gradient fractionation and methyl beta cyclodextrin, a cholesterol-sequestering drug, to demonstrate not only that VP5 copurifies with lipid raft domains in both transfected and infected cells, but also that raft domain integrity is required for BTV assembly. Previously, we showed that BTV nonstructural protein 3 (NS3) interacts with VP2 and also with cellular exocytosis and ESCRT pathway proteins, indicating its involvement in virus egress (A. R. Beaton, J. Rodriguez, Y. K. Reddy, and P. Roy, Proc. Natl. Acad. Sci. USA 99:13154-13159, 2002; C. Wirblich, B. Bhattacharya, and P. Roy J. Virol. 80:460-473, 2006). Here, we show by pull-down and confocal analysis that NS3 also interacts with VP5. Further, a conserved membrane-docking domain similar to the motif in synaptotagmin, a protein belonging to the SNARE (soluble N-ethylmaleimide-sensitive fusion attachment protein receptor) family was identified in the VP5 sequence. By site-directed mutagenesis, followed by flotation and confocal analyses, we demonstrated that raft association of VP5 depends on this domain. Together, these results indicate that VP5 possesses an autonomous signal for its membrane targeting and that the interaction of VP5 with membrane-associated NS3 might play an important role in virus assembly.
Figures
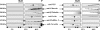



Similar articles
-
A viral nonstructural protein regulates bluetongue virus trafficking and release.J Virol. 2009 Jul;83(13):6806-16. doi: 10.1128/JVI.00263-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369335 Free PMC article.
-
Bluetongue virus coat protein VP2 contains sialic acid-binding domains, and VP5 resembles enveloped virus fusion proteins.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6292-7. doi: 10.1073/pnas.0913403107. Epub 2010 Mar 23. Proc Natl Acad Sci U S A. 2010. PMID: 20332209 Free PMC article.
-
Cellular phosphoinositides and the maturation of bluetongue virus, a non-enveloped capsid virus.Virol J. 2013 Mar 5;10:73. doi: 10.1186/1743-422X-10-73. Virol J. 2013. PMID: 23497128 Free PMC article.
-
Bluetongue virus capsid assembly and maturation.Viruses. 2014 Aug 21;6(8):3250-70. doi: 10.3390/v6083250. Viruses. 2014. PMID: 25196482 Free PMC article. Review.
-
Bluetongue virus assembly and exit pathways.Adv Virus Res. 2020;108:249-273. doi: 10.1016/bs.aivir.2020.08.002. Epub 2020 Sep 16. Adv Virus Res. 2020. PMID: 33837718 Free PMC article. Review.
Cited by
-
Lipids in host-pathogen interactions: pathogens exploit the complexity of the host cell lipidome.Prog Lipid Res. 2010 Jan;49(1):1-26. doi: 10.1016/j.plipres.2009.07.003. Epub 2009 Jul 26. Prog Lipid Res. 2010. PMID: 19638285 Free PMC article. Review.
-
Trafficking of bluetongue virus visualized by recovery of tetracysteine-tagged virion particles.J Virol. 2014 Nov;88(21):12656-68. doi: 10.1128/JVI.01815-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142589 Free PMC article.
-
Turnover Rate of NS3 Proteins Modulates Bluetongue Virus Replication Kinetics in a Host-Specific Manner.J Virol. 2015 Oct;89(20):10467-81. doi: 10.1128/JVI.01541-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246581 Free PMC article.
-
Function of membrane rafts in viral lifecycles and host cellular response.Biochem Res Int. 2011;2011:245090. doi: 10.1155/2011/245090. Epub 2011 Dec 7. Biochem Res Int. 2011. PMID: 22191032 Free PMC article.
-
Structural Domains of the Herpes Simplex Type 1 gD Protein that Restrict HIV-1 Particle Infectivity.J Virol. 2021 Mar 25;95(8):e02355-20. doi: 10.1128/JVI.02355-20. Epub 2021 Feb 3. J Virol. 2021. PMID: 33536165 Free PMC article.
References
-
- Baumgartner, S., J. T. Littleton, K. Broadie, M. A. Bhat, R. Harbecke, J. A. Lengyel, R. Chiquet-Ehrismann, A. Prokop, and H. J. Bellen. 1996. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 871059-1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

