Foot-and-mouth disease virus, but not bovine enterovirus, targets the host cell cytoskeleton via the nonstructural protein 3Cpro
- PMID: 18753210
- PMCID: PMC2573224
- DOI: 10.1128/JVI.00907-08
Foot-and-mouth disease virus, but not bovine enterovirus, targets the host cell cytoskeleton via the nonstructural protein 3Cpro
Abstract
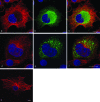
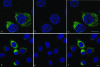
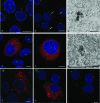
Foot-and-mouth disease virus (FMDV), a member of the Picornaviridae, is a pathogen of cloven-hoofed animals and causes a disease of major economic importance. Picornavirus-infected cells show changes in cell morphology and rearrangement of cytoplasmic membranes, which are a consequence of virus replication. We show here, by confocal immunofluorescence and electron microscopy, that the changes in morphology of FMDV-infected cells involve changes in the distribution of microtubule and intermediate filament components during infection. Despite the continued presence of centrosomes in infected cells, there is a loss of tethering of microtubules to the microtubule organizing center (MTOC) region. Loss of labeling for gamma-tubulin, but not pericentrin, from the MTOC suggests a targeting of gamma-tubulin (or associated proteins) rather than a total breakdown in MTOC structure. The identity of the FMDV protein(s) responsible was determined by the expression of individual viral nonstructural proteins and their precursors in uninfected cells. We report that the only viral nonstructural protein able to reproduce the loss of gamma-tubulin from the MTOC and the loss of integrity of the microtubule system is FMDV 3C(pro). In contrast, infection of cells with another picornavirus, bovine enterovirus, did not affect gamma-tubulin distribution, and the microtubule network remained relatively unaffected.
Figures



Similar articles
-
Analysis of the interaction between host factor Sam68 and viral elements during foot-and-mouth disease virus infections.Virol J. 2015 Dec 23;12:224. doi: 10.1186/s12985-015-0452-8. Virol J. 2015. PMID: 26695943 Free PMC article.
-
The Cellular Chaperone Heat Shock Protein 90 Is Required for Foot-and-Mouth Disease Virus Capsid Precursor Processing and Assembly of Capsid Pentamers.J Virol. 2018 Feb 12;92(5):e01415-17. doi: 10.1128/JVI.01415-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29212943 Free PMC article.
-
Potent small molecule inhibitors against the 3C protease of foot-and-mouth disease virus.Microbiol Spectr. 2024 Apr 2;12(4):e0337223. doi: 10.1128/spectrum.03372-23. Epub 2024 Mar 11. Microbiol Spectr. 2024. PMID: 38466127 Free PMC article.
-
Foot-and-mouth disease virus 3C protease: recent structural and functional insights into an antiviral target.Int J Biochem Cell Biol. 2007;39(1):1-6. doi: 10.1016/j.biocel.2006.07.006. Epub 2006 Aug 14. Int J Biochem Cell Biol. 2007. PMID: 16979372 Free PMC article. Review.
-
Interplay of foot and mouth disease virus with cell-mediated and humoral immunity of host.Rev Med Virol. 2018 Mar;28(2). doi: 10.1002/rmv.1966. Epub 2017 Dec 28. Rev Med Virol. 2018. PMID: 29282795 Review.
Cited by
-
Cell susceptibility to baculovirus transduction and echovirus infection is modified by protein kinase C phosphorylation and vimentin organization.J Virol. 2013 Sep;87(17):9822-35. doi: 10.1128/JVI.01004-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824807 Free PMC article.
-
Exploring IRES region accessibility by interference of foot-and-mouth disease virus infectivity.PLoS One. 2012;7(7):e41382. doi: 10.1371/journal.pone.0041382. Epub 2012 Jul 18. PLoS One. 2012. PMID: 22815996 Free PMC article.
-
RSV-induced expanded ciliated cells contribute to bronchial wall thickening.Virus Res. 2023 Apr 2;327:199060. doi: 10.1016/j.virusres.2023.199060. Epub 2023 Feb 14. Virus Res. 2023. PMID: 36746339 Free PMC article.
-
Cellular Vimentin Interacts with Foot-and-Mouth Disease Virus Nonstructural Protein 3A and Negatively Modulates Viral Replication.J Virol. 2020 Jul 30;94(16):e00273-20. doi: 10.1128/JVI.00273-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32493819 Free PMC article.
-
Foot-and-mouth disease virus utilizes an autophagic pathway during viral replication.Virology. 2011 Feb 5;410(1):142-50. doi: 10.1016/j.virol.2010.10.042. Epub 2010 Nov 26. Virology. 2011. PMID: 21112602 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/I/00001151/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 49/C14570/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 49/CO7867/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 201/S14654/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001212/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

