Molecular identity and functional properties of a novel T-type Ca2+ channel cloned from the sensory epithelia of the mouse inner ear
- PMID: 18753322
- PMCID: PMC2576198
- DOI: 10.1152/jn.90707.2008
Molecular identity and functional properties of a novel T-type Ca2+ channel cloned from the sensory epithelia of the mouse inner ear
Abstract
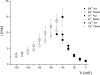
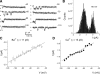
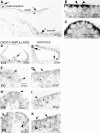
The molecular identity of non-Cav1.3 channels in auditory and vestibular hair cells has remained obscure, yet the evidence in support of their roles to promote diverse Ca2+-dependent functions is indisputable. Recently, a transient Cav3.1 current that serves as a functional signature for the development and regeneration of hair cells has been identified in the chicken basilar papilla. The Cav3.1 current promotes spontaneous activity of the developing hair cell, which may be essential for synapse formation. Here, we have isolated and sequenced the full-length complementary DNA of a distinct isoform of Cav3.1 in the mouse inner ear. The channel is derived from alternative splicing of exon14, exon25A, exon34, and exon35. Functional expression of the channel in Xenopus oocytes yielded Ca2+ currents, which have a permeation phenotype consistent with T-type channels. However, unlike most multiion channels, the T-type channel does not exhibit the anomalous mole fraction effect, possibly reflecting comparable permeation properties of divalent cations. The Cav3.1 channel was expressed in sensory and nonsensory epithelia of the inner ear. Moreover, there are profound changes in the expression levels during development. The differential expression of the channel during development and the pharmacology of the inner ear Cav3.1 channel may have contributed to the difficulties associated with identification of the non-Cav1.3 currents.
Figures








Similar articles
-
The CaV3.1 T-type Ca2+channel contributes to voltage-dependent calcium currents in rat outer hair cells.Brain Res. 2008 Mar 27;1201:68-77. doi: 10.1016/j.brainres.2008.01.058. Epub 2008 Feb 2. Brain Res. 2008. PMID: 18294617
-
Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 channels in mouse auditory hair cells.J Physiol. 2007 Dec 15;585(Pt 3):791-803. doi: 10.1113/jphysiol.2007.142307. Epub 2007 Oct 18. J Physiol. 2007. PMID: 17947313 Free PMC article.
-
Functional impact of alternative splicing of human T-type Cav3.3 calcium channels.J Neurophysiol. 2004 Dec;92(6):3399-407. doi: 10.1152/jn.00498.2004. Epub 2004 Jul 14. J Neurophysiol. 2004. PMID: 15254077
-
Channeling your inner ear potassium: K(+) channels in vestibular hair cells.Hear Res. 2016 Aug;338:40-51. doi: 10.1016/j.heares.2016.01.015. Epub 2016 Feb 4. Hear Res. 2016. PMID: 26836968 Review.
-
Molecular physiology of low-voltage-activated t-type calcium channels.Physiol Rev. 2003 Jan;83(1):117-61. doi: 10.1152/physrev.00018.2002. Physiol Rev. 2003. PMID: 12506128 Review.
Cited by
-
Exocytotic machineries of vestibular type I and cochlear ribbon synapses display similar intrinsic otoferlin-dependent Ca2+ sensitivity but a different coupling to Ca2+ channels.J Neurosci. 2014 Aug 13;34(33):10853-69. doi: 10.1523/JNEUROSCI.0947-14.2014. J Neurosci. 2014. PMID: 25122888 Free PMC article.
-
Epilepsy and Hearing Loss in a Patient with a Rare Heterozygous Variant in the CACNA1H Gene.J Epilepsy Res. 2022 Jun 30;12(1):33-35. doi: 10.14581/jer.22006. eCollection 2022 Jun. J Epilepsy Res. 2022. PMID: 35910327 Free PMC article.
-
Postnatal expression of an apamin-sensitive k(ca) current in vestibular calyx terminals.J Membr Biol. 2011 Nov;244(2):81-91. doi: 10.1007/s00232-011-9400-8. Epub 2011 Nov 5. J Membr Biol. 2011. PMID: 22057903 Free PMC article.
-
Otoprotective effects of ethosuximide in NOD/LtJ mice with age-related hearing loss.Int J Mol Med. 2017 Jul;40(1):146-154. doi: 10.3892/ijmm.2017.3004. Epub 2017 May 26. Int J Mol Med. 2017. PMID: 28560432 Free PMC article.
-
Genetic T-type calcium channelopathies.J Med Genet. 2020 Jan;57(1):1-10. doi: 10.1136/jmedgenet-2019-106163. Epub 2019 Jun 19. J Med Genet. 2020. PMID: 31217264 Free PMC article. Review.
References
-
- Bao H, Wong WH, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J Neurophysiol 90: 155–164, 2003. - PubMed
-
- Barish ME, Mansdorf NB. Development of intracellular calcium responses to depolarization and to kainate and N-methyl-d-aspartate in cultured mouse hippocampal neurons. Brain Res Dev Brain Res 63: 53–61, 1991. - PubMed
-
- Bertolesi GE, Jollimore CA, Shi C, Elbaum L, Denovan-Wright EM, Barnes S, Kelly ME. Regulation of alpha1G T-type calcium channel gene (CACNA1G) expression during neuronal differentiation. Eur J Neurosci 17: 1802–1810, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

