BRI2 inhibits amyloid beta-peptide precursor protein processing by interfering with the docking of secretases to the substrate
- PMID: 18753367
- PMCID: PMC3844774
- DOI: 10.1523/JNEUROSCI.2094-08.2008
BRI2 inhibits amyloid beta-peptide precursor protein processing by interfering with the docking of secretases to the substrate
Abstract
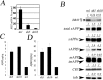
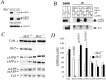
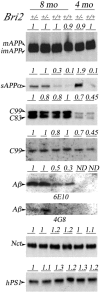
Genetic alterations of amyloid beta-peptide (Abeta) production caused by mutations in the Abeta precursor protein (APP) cause familial Alzheimer's disease (AD). Mutations in BRI2, a gene of undefined function, are linked to familial British and Danish dementias, which are pathologically and clinically similar to Alzheimer's disease. We report that BRI2 is a physiological suppressor of Abeta production. BRI2 restrict docking of gamma-secretase to APP and access of alpha- and beta-secretases to their cleavage APP sequences. Alterations of BRI2 by gene targeting or transgenic expression regulate Abeta levels and AD pathology in mouse models of AD. Competitive inhibition of APP processing by BRI2 may provide a new approach to AD therapy and prevention.
Figures




References
-
- Akiyama H, Kondo H, Arai T, Ikeda K, Kato M, Iseki E, Schwab C, McGeer PL. Expression of BRI, the normal precursor of the amyloid protein of familial British dementia, in human brain. Acta Neuropathol. 2004;107:53–58. - PubMed
-
- Annaert W, De Strooper B. Presenilins: molecular switches between proteolysis and signal transduction. Trends Neurosci. 1999;22:439–443. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Breteler MM, Claus JJ, van Duijn CM, Launer LJ, Hofman A. Epidemiology of Alzheimer's disease. Epidemiol Rev. 1992;14:59–82. - PubMed
-
- Cao X, Südhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
