Manipulating Robo expression in vivo perturbs commissural axon pathfinding in the chick spinal cord
- PMID: 18753371
- PMCID: PMC2886497
- DOI: 10.1523/JNEUROSCI.1479-08.2008
Manipulating Robo expression in vivo perturbs commissural axon pathfinding in the chick spinal cord
Abstract
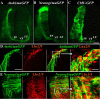
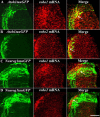
In vertebrate embryos, most spinal commissural axons cross the ventral midline (VM) and project either alongside or significant distances away from the floor plate (FP). The upregulation of repulsive Robo1/2 receptors on postcrossing commissural axons, in mammals, presumably allows these axons to respond to the midline-associated repellents, Slit1-3, facilitating their expulsion from, and prohibiting their reentry into, the FP. Compelling data suggest that Robo3 represses Robo1/2 function on precrossing axons and that Robo1/2 inhibit attractive guidance receptors on postcrossing axons, thereby ensuring that decussated axons are selectively responsive to midline Slits. However, whether Robo1/2 expel decussated commissural axons from the VM and/or prevent their reentry into the FP has not been explicitly established in vivo. Furthermore, some commissural axons do not require Robo1/2 to elaborate appropriate contralateral projections in the mouse spinal cord. Here, we use unilateral in ovo electroporation together with Atoh1 and Neurog1 enhancer elements to visualize, and assess the consequences of manipulating Robo expression on, dl1 and dl2 chick commissural axons. In response to misexpressing a cytoplasmic truncation of Robo1 and/or Robo2, which should block all Robo-ligand interactions, postcrossing commissural axons extend alongside, but do not project away from or reenter the FP. In contrast, misexpression of full-length Robo2 prevents many commissural axons from crossing the VM. Together, these findings support key and selective in vivo roles for Robo receptors in presumably altering the responsiveness of decussated commissural axons and facilitating their expulsion from the VM within the chick spinal cord.
Figures






References
-
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. - PubMed
-
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. - PubMed
-
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen Sonic Hedgehog is an axonal chemoattractant that collaborates with Netrin-1 in midline axon guidance. Cell. 2003;113:11–23. - PubMed
-
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. 2008;58:325–332. - PubMed
-
- Chesnutt C, Niswander L. Plasmid-based short-hairpin RNA interference in the chicken embryo. Genesis. 2004;39:73–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
