Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice
- PMID: 18753372
- PMCID: PMC2561993
- DOI: 10.1523/JNEUROSCI.2077-08.2008
Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice
Abstract
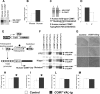
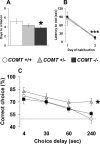
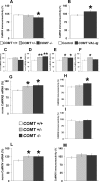
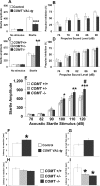
The COMT (catechol-O-methyltransferase) gene has been linked to a spectrum of human phenotypes, including cognition, anxiety, pain sensitivity and psychosis. Doubts about its clinical impact exist, however, because of the complexity of human COMT polymorphism and clinical variability. We generated transgenic mice overexpressing a human COMT-Val polymorphism (Val-tg), and compared them with mice containing a null COMT mutation. Increased COMT enzyme activity in Val-tg mice resulted in disrupted attentional set-shifting abilities, and impaired working and recognition memory, but blunted stress responses and pain sensitivity. Conversely, COMT disruption improved working memory, but increased stress responses and pain sensitivity. Amphetamine ameliorated recognition memory deficits in COMT-Val-tg mice but disrupted it in wild types, illustrating COMT modulation of the inverted-U relationship between cognition and dopamine. COMT-Val-tg mice showed increased prefrontal cortex (PFC) calcium/calmodulin-dependent protein kinase II (CaMKII) levels, whereas COMT deficiency decreased PFC CaMKII but increased PFC CaMKKbeta and CaMKIV levels, suggesting the involvement of PFC CaMK pathways in COMT-regulated cognitive function and adaptive stress responses. Our data indicate a critical role for the COMT gene in an apparent evolutionary trade-off between cognitive and affective functions.
Conflict of interest statement
The authors declare that they have no financial conflict of interest.
Figures



References
-
- Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. - PubMed
-
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem. 1998;273:31880–31889. - PubMed
-
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–192. - PubMed
-
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 2001;153:353–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
