Suppression of mutant Huntingtin aggregate formation by Cdk5/p35 through the effect on microtubule stability
- PMID: 18753376
- PMCID: PMC6670830
- DOI: 10.1523/JNEUROSCI.0973-08.2008
Suppression of mutant Huntingtin aggregate formation by Cdk5/p35 through the effect on microtubule stability
Abstract
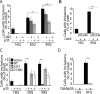
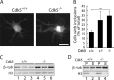
Huntington's disease (HD) is a polyglutamine [poly(Q)] disease with an expanded poly(Q) stretch in the N terminus of the huntingtin protein (htt). A major pathological feature of HD neurons is inclusion bodies, detergent-insoluble aggregates composed of poly(Q)-expanded mutant htt (mhtt). Misfolding of mhtt is thought to confer a toxic property via formation of aggregates. Although toxic molecular species are still debated, it is important to clarify the aggregation mechanism to understand the pathogenesis of mhtt. We show Cdk5/p35 suppresses the formation of mhtt inclusion bodies in cell lines and primary neurons. Although we expressed the N-terminal exon 1 fragment of htt lacking phosphorylation sites for Cdk5 in COS-7 cells, the kinase activity of Cdk5 was required for the suppression. Furthermore, Cdk5/p35 suppressed inclusion formation of atrophin-1, another poly(Q) protein, raising the possibility that Cdk5/p35 generally suppresses inclusion formation of poly(Q) proteins. Microtubules (MTs) were a downstream component of Cdk5/p35 in the suppression of inclusion formation; Cdk5/p35 disrupted MTs, which were required for the formation of inclusions. Moreover, stabilization of MTs by Taxol induced inclusions even with overexpression of Cdk5/p35. The formation of inclusions was also regulated by manipulating the Cdk5/p35 activity in primary rat or mouse cortical neuron cultures. These results indicate that Cdk5-dependent regulation of MT organization is involved in the development of aggregate formation and subsequent pathogenesis of poly(Q) diseases. This Cdk5 inhibition of htt aggregates is a novel mechanism different from htt phosphorylation and interaction with Cdk5 reported previously (Luo et al., 2005; Anne et al., 2007).
Figures




Similar articles
-
Cdk5 phosphorylation of huntingtin reduces its cleavage by caspases: implications for mutant huntingtin toxicity.J Cell Biol. 2005 May 23;169(4):647-56. doi: 10.1083/jcb.200412071. J Cell Biol. 2005. PMID: 15911879 Free PMC article.
-
High efficiency adenovirus-mediated expression of truncated N-terminal huntingtin fragment (htt552) in primary rat astrocytes.Acta Biochim Biophys Sin (Shanghai). 2009 Apr;41(4):325-34. doi: 10.1093/abbs/gmp021. Acta Biochim Biophys Sin (Shanghai). 2009. PMID: 19352548
-
14-3-3zeta is indispensable for aggregate formation of polyglutamine-expanded huntingtin protein.Neurosci Lett. 2008 Jan 24;431(1):45-50. doi: 10.1016/j.neulet.2007.11.018. Epub 2007 Nov 17. Neurosci Lett. 2008. PMID: 18078716
-
The selective vulnerability of nerve cells in Huntington's disease.Neuropathol Appl Neurobiol. 2001 Feb;27(1):1-21. doi: 10.1046/j.0305-1846.2001.00299.x. Neuropathol Appl Neurobiol. 2001. PMID: 11298997 Review.
-
Huntingtin processing in pathogenesis of Huntington disease.Acta Pharmacol Sin. 2004 Oct;25(10):1243-9. Acta Pharmacol Sin. 2004. PMID: 15456523 Review.
Cited by
-
Analysis of Huntington's Disease Modifiers Using the Hyperbolic Mapping of the Protein Interaction Network.Int J Mol Sci. 2022 May 23;23(10):5853. doi: 10.3390/ijms23105853. Int J Mol Sci. 2022. PMID: 35628660 Free PMC article.
-
Mutant Huntingtin Protein Interaction Map Implicates Dysregulation of Multiple Cellular Pathways in Neurodegeneration of Huntington's Disease.J Huntingtons Dis. 2022;11(3):243-267. doi: 10.3233/JHD-220538. J Huntingtons Dis. 2022. PMID: 35871359 Free PMC article.
-
Exploiting Post-mitotic Yeast Cultures to Model Neurodegeneration.Front Mol Neurosci. 2018 Nov 2;11:400. doi: 10.3389/fnmol.2018.00400. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30450036 Free PMC article. Review.
-
Actin interaction and regulation of cyclin-dependent kinase 5/p35 complex activity.J Neurochem. 2011 Jan;116(2):192-204. doi: 10.1111/j.1471-4159.2010.06824.x. J Neurochem. 2011. PMID: 20492361 Free PMC article.
-
Roscovitine, a CDK Inhibitor, Reduced Neuronal Toxicity of mHTT by Targeting HTT Phosphorylation at S1181 and S1201 In Vitro.Int J Mol Sci. 2024 Nov 16;25(22):12315. doi: 10.3390/ijms252212315. Int J Mol Sci. 2024. PMID: 39596381 Free PMC article.
References
-
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. - PubMed
-
- Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. Global changes to the ubiquitin system in Huntington's disease. Nature. 2007;448:704–708. - PubMed
-
- Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat Rev Neurosci. 2005;6:919–930. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
