Multiple mechanisms for integrating proprioceptive inputs that converge on the same motor pattern-generating network
- PMID: 18753383
- PMCID: PMC6670814
- DOI: 10.1523/JNEUROSCI.2095-08.2008
Multiple mechanisms for integrating proprioceptive inputs that converge on the same motor pattern-generating network
Abstract
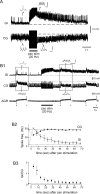
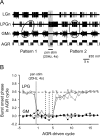
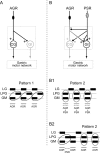
Movement-derived sensory feedback adapts centrally generated motor programs to changing behavioral demands. Motor circuit output may also be shaped by distinct proprioceptive systems with different central actions, although little is known about the integrative processes by which such convergent sensorimotor regulation occurs. Here, we explore the combined actions of two previously identified proprioceptors on the gastric mill motor network in the lobster stomatogastric nervous system. Both mechanoreceptors [anterior gastric receptor (AGR) and posterior stomach receptor (PSR)] access the gastric circuit via the same pair of identified projection interneurons that either excite [commissural gastric (CG)] or inhibit [gastric inhibitor (GI)] different subsets of gastric network neurons. Mechanosensory information from the two receptors is integrated upstream to the gastric circuit at two levels: (1) postsynaptically, where both receptors excite the GI neuron while exerting opposing effects on the CG neuron, and (2) presynaptically, where PSR reduces AGR's excitation of the CG projection neuron. Concomitantly PSR selectively enhances AGR's activation of the GI neuron, possibly also via a presynaptic action. PSR's influences also far outlast its transient synaptic effects, indicating the additional involvement of modulatory processes. Consequently, PSR activation causes parallel input from AGR to be conveyed preferentially via the GI interneuron, resulting in a prolonged switch in the pattern of gastric circuit output. Therefore, via a combination of short- and long-lasting, presynaptic and postsynaptic actions, one proprioceptive system is able to promote its impact on a target motor network by biasing the access of a different sensory system to the same circuit.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
