A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing
- PMID: 18753386
- PMCID: PMC2737175
- DOI: 10.1523/JNEUROSCI.1810-08.2008
A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing
Abstract
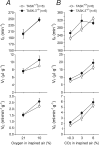
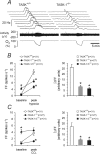
Acid-sensitive K+ channels of the tandem P-domain K+-channel family (TASK-1 and TASK-3) have been implicated in peripheral and central respiratory chemosensitivity; however, because of the lack of decisive pharmacological agents, the final proof of the role of the TASK channel in the chemosensory control of breathing has been missing. In the mouse, TASK-1 and TASK-3 channels are dispensable for central respiratory chemosensitivity (Mulkey et al., 2007). Here, we have used knock-out animals to determine whether TASK-1 and TASK-3 channels play a role in the carotid body function and chemosensory control of breathing exerted by the carotid body chemoreceptors. Ventilatory responses to hypoxia (10% O2 in inspired air) and moderate normoxic hypercapnia (3-6% CO2 in inspired air) were significantly reduced in TASK-1 knock-out mice. In contrast, TASK-3-deficient mice showed responses to both stimuli that were similar to those developed by their wild-type counterparts. TASK-1 channel deficiency resulted in a marked reduction of the hypoxia (by 49%)- and CO2 (by 68%)-evoked increases in the carotid sinus nerve chemoafferent discharge recorded in the in vitro superfused carotid body/carotid sinus nerve preparations. Deficiency in both TASK-1 and TASK-3 channels increased baseline chemoafferent activity but did not cause a further reduction of the carotid body chemosensory responses. These observations provide direct evidence that TASK-1 channels contribute significantly to the increases in the carotid body chemoafferent discharge in response to a decrease in arterial P(O2) or an increase in P(CO2)/[H+]. TASK-1 channels therefore play a key role in the control of ventilation by peripheral chemoreceptors.
Figures


References
-
- Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–174. - PubMed
-
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
