Stress-activated genomic expression changes serve a preparative role for impending stress in yeast
- PMID: 18753408
- PMCID: PMC2575158
- DOI: 10.1091/mbc.e07-07-0680
Stress-activated genomic expression changes serve a preparative role for impending stress in yeast
Abstract
Yeast cells respond to stress by mediating condition-specific gene expression changes and by mounting a common response to many stresses, called the environmental stress response (ESR). Giaever et al. previously revealed poor correlation between genes whose expression changes in response to acute stress and genes required to survive that stress, raising question about the role of stress-activated gene expression. Here we show that gene expression changes triggered by a single dose of stress are not required to survive that stimulus but rather serve a protective role against future stress. We characterized the increased resistance to severe stress in yeast preexposed to mild stress. This acquired stress resistance is dependent on protein synthesis during mild-stress treatment and requires the "general-stress" transcription factors Msn2p and/or Msn4p that regulate induction of many ESR genes. However, neither protein synthesis nor Msn2/4p is required for basal tolerance of a single dose of stress, despite the substantial expression changes triggered by each condition. Using microarrays, we show that Msn2p and Msn4p play nonredundant and condition-specific roles in gene-expression regulation, arguing against a generic general-stress function. This work highlights the importance of condition-specific responses in acquired stress resistance and provides new insights into the role of the ESR.
Figures


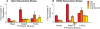
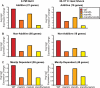
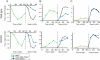
Comment in
- Mol Biol Cell. 19:4545.
References
-
- Amoros M., Estruch F. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol. Microbiol. 2001;39:1523–1532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

