Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor
- PMID: 18753623
- PMCID: PMC2526098
- DOI: 10.1073/pnas.0806312105
Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor
Abstract
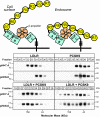
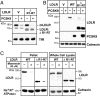
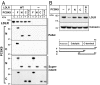
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a secreted protein that controls plasma LDL cholesterol levels by posttranslational regulation of the LDL receptor (LDLR). Previously, we showed that PCSK9 binds specifically to an EGF-like repeat (EGF-A) in LDLR and reroutes the receptor from endosomes to lysosomes rather than to the cell surface. Here, we defined the regions in LDLR and PCSK9 that are required for receptor degradation and examined the relationship between PCSK9 binding and LDLR conformation. Addition of PCSK9 to cultured hepatocytes promoted degradation of WT LDLR and of receptors lacking up to four ligand binding domains, EGF-B or the clustered O-linked sugar region. In contrast, LDLRs lacking the entire ligand binding domain or the beta-propeller domain failed to be degraded, although they bound and internalized PCSK9. Using gel filtration chromatography, we assessed the effects of PCSK9 binding on an acid-dependent conformational change that happens in the extracellular domain of the LDLR. Although PCSK9 prevented the reduction in hydrodynamic radius of the receptor that occurs at a reduced pH, the effect was not sufficient for LDLR degradation. A truncated version of PCSK9 containing the prodomain and the catalytic domain, but not the C-terminal domain, bound the receptor but did not stimulate LDLR degradation. Thus, domains in both the LDLR and PCSK9 that are not required for binding (or internalization) are essential for PCSK9-mediated degradation of the LDLR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation.J Biol Chem. 2007 Jun 22;282(25):18602-18612. doi: 10.1074/jbc.M702027200. Epub 2007 Apr 23. J Biol Chem. 2007. PMID: 17452316
-
Characterization of the role of EGF-A of low density lipoprotein receptor in PCSK9 binding.J Lipid Res. 2013 Dec;54(12):3345-57. doi: 10.1194/jlr.M041129. Epub 2013 Oct 8. J Lipid Res. 2013. PMID: 24103783 Free PMC article.
-
Molecular basis for LDL receptor recognition by PCSK9.Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):1820-5. doi: 10.1073/pnas.0712064105. Epub 2008 Feb 4. Proc Natl Acad Sci U S A. 2008. PMID: 18250299 Free PMC article.
-
Sorting an LDL receptor with bound PCSK9 to intracellular degradation.Atherosclerosis. 2014 Nov;237(1):76-81. doi: 10.1016/j.atherosclerosis.2014.08.038. Epub 2014 Sep 2. Atherosclerosis. 2014. PMID: 25222343 Review.
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
Cited by
-
Inducible phospholipid transfer protein deficiency ameliorates atherosclerosis.Atherosclerosis. 2021 May;324:9-17. doi: 10.1016/j.atherosclerosis.2021.03.011. Epub 2021 Mar 13. Atherosclerosis. 2021. PMID: 33798923 Free PMC article.
-
Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1).PLoS One. 2013 May 13;8(5):e64145. doi: 10.1371/journal.pone.0064145. Print 2013. PLoS One. 2013. PMID: 23675525 Free PMC article.
-
Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9.J Biol Chem. 2012 Mar 30;287(14):11090-7. doi: 10.1074/jbc.M111.319764. Epub 2012 Jan 31. J Biol Chem. 2012. PMID: 22294692 Free PMC article.
-
The PCSK9 decade.J Lipid Res. 2012 Dec;53(12):2515-24. doi: 10.1194/jlr.R026658. Epub 2012 Jul 17. J Lipid Res. 2012. PMID: 22811413 Free PMC article. Review.
-
Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications.Front Cardiovasc Med. 2021 Oct 15;8:764038. doi: 10.3389/fcvm.2021.764038. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34782856 Free PMC article. Review.
References
-
- Goldstein J, Hobbs H, Brown M, Scriver C, Beaudet A, Sly W, Valle D. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw Hill; 2001. pp. 2863–2913.
-
- Innerarity TL, et al. Familial defective apolipoprotein B-100: A mutation of apolipoprotein B that causes hypercholesterolemia. J Lipid Res. 1990;31:1337–1349. - PubMed
-
- Garcia CK, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science. 2001;292:1394–1398. - PubMed
-
- Abifadel M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

