Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke
- PMID: 18753638
- PMCID: PMC2714259
- DOI: 10.1056/NEJMoa0805002
Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke
Abstract
Background: Recurrent stroke is a frequent, disabling event after ischemic stroke. This study compared the efficacy and safety of two antiplatelet regimens--aspirin plus extended-release dipyridamole (ASA-ERDP) versus clopidogrel.
Methods: In this double-blind, 2-by-2 factorial trial, we randomly assigned patients to receive 25 mg of aspirin plus 200 mg of extended-release dipyridamole twice daily or to receive 75 mg of clopidogrel daily. The primary outcome was first recurrence of stroke. The secondary outcome was a composite of stroke, myocardial infarction, or death from vascular causes. Sequential statistical testing of noninferiority (margin of 1.075), followed by superiority testing, was planned.
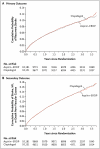
Results: A total of 20,332 patients were followed for a mean of 2.5 years. Recurrent stroke occurred in 916 patients (9.0%) receiving ASA-ERDP and in 898 patients (8.8%) receiving clopidogrel (hazard ratio, 1.01; 95% confidence interval [CI], 0.92 to 1.11). The secondary outcome occurred in 1333 patients (13.1%) in each group (hazard ratio for ASA-ERDP, 0.99; 95% CI, 0.92 to 1.07). There were more major hemorrhagic events among ASA-ERDP recipients (419 [4.1%]) than among clopidogrel recipients (365 [3.6%]) (hazard ratio, 1.15; 95% CI, 1.00 to 1.32), including intracranial hemorrhage (hazard ratio, 1.42; 95% CI, 1.11 to 1.83). The net risk of recurrent stroke or major hemorrhagic event was similar in the two groups (1194 ASA-ERDP recipients [11.7%], vs. 1156 clopidogrel recipients [11.4%]; hazard ratio, 1.03; 95% CI, 0.95 to 1.11).
Conclusions: The trial did not meet the predefined criteria for noninferiority but showed similar rates of recurrent stroke with ASA-ERDP and with clopidogrel. There is no evidence that either of the two treatments was superior to the other in the prevention of recurrent stroke. (ClinicalTrials.gov number, NCT00153062.)
2008 Massachusetts Medical Society
Figures


Comment in
-
Stroke prevention--insights from incoherence.N Engl J Med. 2008 Sep 18;359(12):1287-9. doi: 10.1056/NEJMe0806806. Epub 2008 Aug 27. N Engl J Med. 2008. PMID: 18753641 No abstract available.
-
Is combination therapy with aspirin and extended-release dipyridamole more effective than clopidogrel for preventing recurrent stroke?Curr Cardiol Rep. 2009 Jan;11(1):3. doi: 10.1007/s11886-009-0001-4. Curr Cardiol Rep. 2009. PMID: 19091168 No abstract available.
-
Is aspirin plus dipyridamole as effective as clopidogrel for preventing recurrent stroke?J Fam Pract. 2008 Dec;57(12):781. J Fam Pract. 2008. PMID: 19097339 No abstract available.
-
ACP Journal Club. Aspirin plus extended-release dipyridamole and clopidogrel were similarly effective for preventing recurrent stroke.Ann Intern Med. 2009 Feb 17;150(4):JC2-8, JC2-9. doi: 10.7326/0003-4819-150-4-200902170-02008. Ann Intern Med. 2009. PMID: 19238606 No abstract available.
-
Aspirin plus extended-release dipyridamole and clopidogrel were similarly effective for preventing recurrent stroke.Evid Based Med. 2009 Apr;14(2):46-7. doi: 10.1136/ebm.14.2.46. Evid Based Med. 2009. PMID: 19332602 No abstract available.
References
-
- Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–6. - PubMed
-
- Gent M, Blakely JA, Easton JD, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet. 1989;1:1215–20. - PubMed
-
- Hass WK, Easton JD, Adams HP, Jr, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. N Engl J Med. 1989;321:501–7. - PubMed
-
- A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE) Lancet. 1996;348:1329–39. - PubMed
-
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
