Telmisartan to prevent recurrent stroke and cardiovascular events
- PMID: 18753639
- PMCID: PMC2714258
- DOI: 10.1056/NEJMoa0804593
Telmisartan to prevent recurrent stroke and cardiovascular events
Abstract
Background: Prolonged lowering of blood pressure after a stroke reduces the risk of recurrent stroke. In addition, inhibition of the renin-angiotensin system in high-risk patients reduces the rate of subsequent cardiovascular events, including stroke. However, the effect of lowering of blood pressure with a renin-angiotensin system inhibitor soon after a stroke has not been clearly established. We evaluated the effects of therapy with an angiotensin-receptor blocker, telmisartan, initiated early after a stroke.
Methods: In a multicenter trial involving 20,332 patients who recently had an ischemic stroke, we randomly assigned 10,146 to receive telmisartan (80 mg daily) and 10,186 to receive placebo. The primary outcome was recurrent stroke. Secondary outcomes were major cardiovascular events (death from cardiovascular causes, recurrent stroke, myocardial infarction, or new or worsening heart failure) and new-onset diabetes.
Results: The median interval from stroke to randomization was 15 days. During a mean follow-up of 2.5 years, the mean blood pressure was 3.8/2.0 mm Hg lower in the telmisartan group than in the placebo group. A total of 880 patients (8.7%) in the telmisartan group and 934 patients (9.2%) in the placebo group had a subsequent stroke (hazard ratio in the telmisartan group, 0.95; 95% confidence interval [CI], 0.86 to 1.04; P=0.23). Major cardiovascular events occurred in 1367 patients (13.5%) in the telmisartan group and 1463 patients (14.4%) in the placebo group (hazard ratio, 0.94; 95% CI, 0.87 to 1.01; P=0.11). New-onset diabetes occurred in 1.7% of the telmisartan group and 2.1% of the placebo group (hazard ratio, 0.82; 95% CI, 0.65 to 1.04; P=0.10).
Conclusions: Therapy with telmisartan initiated soon after an ischemic stroke and continued for 2.5 years did not significantly lower the rate of recurrent stroke, major cardiovascular events, or diabetes. (ClinicalTrials.gov number, NCT00153062.)
2008 Massachusetts Medical Society
Figures
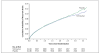
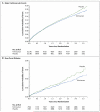
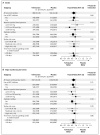
Comment in
-
Stroke prevention--insights from incoherence.N Engl J Med. 2008 Sep 18;359(12):1287-9. doi: 10.1056/NEJMe0806806. Epub 2008 Aug 27. N Engl J Med. 2008. PMID: 18753641 No abstract available.
-
Telmisartan for prevention of cardiovascular events.N Engl J Med. 2009 Jan 15;360(3):302; author reply 303. doi: 10.1056/NEJMc082129. N Engl J Med. 2009. PMID: 19144949 No abstract available.
-
Telmisartan for prevention of cardiovascular events.N Engl J Med. 2009 Jan 15;360(3):302-3; author reply 303. N Engl J Med. 2009. PMID: 19148963 No abstract available.
-
Telmisartan for prevention of cardiovascular events.N Engl J Med. 2009 Jan 15;360(3):303; author reply 303. N Engl J Med. 2009. PMID: 19148964 No abstract available.
-
ACP Journal Club. Telmisartan did not prevent recurrent stroke or major cardiovascular events.Ann Intern Med. 2009 Feb 17;150(4):JC2-8, JC2-9. doi: 10.7326/0003-4819-150-4-200902170-02009. Ann Intern Med. 2009. PMID: 19238607 No abstract available.
-
Telmisartan did not prevent recurrent stroke or major cardiovascular events.Evid Based Med. 2009 Apr;14(2):46-7. doi: 10.1136/ebm.14.2.47. Evid Based Med. 2009. PMID: 19332603 No abstract available.
-
Is telmisartan effective for stroke prevention?Am Fam Physician. 2009 Nov 15;80(10):1042. Am Fam Physician. 2009. PMID: 19904889 No abstract available.
References
-
- Murray CJL, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. - PubMed
-
- Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. - PubMed
-
- PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. Errata, Lancet 2001;358: 1556, 2002;359:2120. - PubMed
-
- Lonn EM, Yusuf S, Jha P, et al. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994;90:2056–69. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
