A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy
- PMID: 18753646
- PMCID: PMC2803083
- DOI: 10.1056/NEJMoa0801187
A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy
Abstract
Background: Research suggests that fetal exposure to magnesium sulfate before preterm birth might reduce the risk of cerebral palsy.
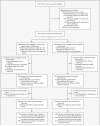
Methods: In this multicenter, placebo-controlled, double-blind trial, we randomly assigned women at imminent risk for delivery between 24 and 31 weeks of gestation to receive magnesium sulfate, administered intravenously as a 6-g bolus followed by a constant infusion of 2 g per hour, or matching placebo. The primary outcome was the composite of stillbirth or infant death by 1 year of corrected age or moderate or severe cerebral palsy at or beyond 2 years of corrected age.
Results: A total of 2241 women underwent randomization. The baseline characteristics were similar in the two groups. Follow-up was achieved for 95.6% of the children. The rate of the primary outcome was not significantly different in the magnesium sulfate group and the placebo group (11.3% and 11.7%, respectively; relative risk, 0.97; 95% confidence interval [CI], 0.77 to 1.23). However, in a prespecified secondary analysis, moderate or severe cerebral palsy occurred significantly less frequently in the magnesium sulfate group (1.9% vs. 3.5%; relative risk, 0.55; 95% CI, 0.32 to 0.95). The risk of death did not differ significantly between the groups (9.5% vs. 8.5%; relative risk, 1.12; 95% CI, 0.85 to 1.47). No woman had a life-threatening event.
Conclusions: Fetal exposure to magnesium sulfate before anticipated early preterm delivery did not reduce the combined risk of moderate or severe cerebral palsy or death, although the rate of cerebral palsy was reduced among survivors. (ClinicalTrials.gov number, NCT00014989.)
2008 Massachusetts Medical Society
Figures
Comment in
-
Antenatal magnesium sulfate for neuroprotection before preterm birth?N Engl J Med. 2008 Aug 28;359(9):962-4. doi: 10.1056/NEJMe0805018. N Engl J Med. 2008. PMID: 18753653 No abstract available.
-
Magnesium sulfate for the prevention of cerebral palsy.N Engl J Med. 2009 Jan 8;360(2):189-90; author reply 190. doi: 10.1056/NEJMc081995. N Engl J Med. 2009. PMID: 19129535 No abstract available.
-
Magnesium treatment of mothers may decrease the incidence of cerebral palsy in at-risk infants.J Pediatr. 2009 Jan;154(1):151-2. doi: 10.1016/j.jpeds.2008.10.026. J Pediatr. 2009. PMID: 19187747 No abstract available.
-
Antenatal magnesium for preterm delivery reduces risk of cerebral palsy among surviving very preterm infants.Acta Paediatr. 2018 Jan;107(1):175. doi: 10.1111/apa.14116. Epub 2017 Oct 30. Acta Paediatr. 2018. PMID: 29083069 No abstract available.
References
-
- Executive Committee for the Definition of Cerebral Palsy Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–6. - PubMed
-
- Kuban KCK, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188–95. - PubMed
-
- Cummins SK, Nelson KB, Grether JK, Veile EM. Cerebral palsy in four northern California counties, births 1983 through 1985. J Pediatr. 1993;123:230–7. - PubMed
-
- Robertson CMT, Watt M-J, Yasui Y. Changes in the prevalence of cerebral palsy for children born very prematurely in a population-based program over 30 years. JAMA. 2007;297:2733–40. - PubMed
-
- Tommiska V, Heinonen K, Lehtonen L, et al. No improvement in outcome of nationwide extremely low birthweight infant populations between 1996-1997 and 1999-2000. Pediatrics. 2007;119:29–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U01 HD019897/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- HD27861/HD/NICHD NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- HD21414/HD/NICHD NIH HHS/United States
- U10 HD027905/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD53907/HD/NICHD NIH HHS/United States
- HD34210/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD034122/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical