Mercury vapor release from broken compact fluorescent lamps and in situ capture by new nanomaterial sorbents
- PMID: 18754507
- PMCID: PMC2646878
- DOI: 10.1021/es8004392
Mercury vapor release from broken compact fluorescent lamps and in situ capture by new nanomaterial sorbents
Abstract
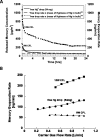
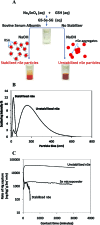
The projected increase in the use of compact fluorescent lamps (CFLs) motivates the development of methods to manage consumer exposure to mercury and its environmental release at the end of lamp life. This work characterizes the time-resolved release of mercury vapor from broken CFLs and from underlying substrates after removal of glass fragments to simulate cleanup. In new lamps, mercury vapor is released gradually in amounts that reach 1.3 mg or 30% of the total lamp inventory after four days. Similar time profiles but smaller amounts are released from spent lamps or from underlying substrates. Nanoscale formulations of S, Se, Cu, Ni, Zn, Ag, and WS2 are evaluated for capture of Hg vapor under these conditions and compared to conventional microscale formulations. Adsorption capacities range over 7 orders of magnitude, from 0.005 (Zn micropowder) to 188 000 microg/g (unstabilized nano-Se), depending on sorbent chemistry and particle size. Nanosynthesis offers clear advantages for most sorbent chemistries. Unstabilized nano-selenium in two forms (dry powder and impregnated cloth) was successfully used in a proof-of-principle test for the in situ, real-time suppression of Hg vapor escape following CFL fracture.
Figures



Similar articles
-
A nano-selenium reactive barrier approach for managing mercury over the life-cycle of compact fluorescent lamps.Environ Sci Technol. 2009 Aug 1;43(15):5915-20. doi: 10.1021/es9013097. Environ Sci Technol. 2009. PMID: 19731697 Free PMC article.
-
Preventing mercury vapor release from broken fluorescent lamps during shipping.J Air Waste Manag Assoc. 2009 Mar;59(3):266-72. doi: 10.3155/1047-3289.59.3.266. J Air Waste Manag Assoc. 2009. PMID: 19320265
-
Determination of mercury distribution inside spent compact fluorescent lamps by atomic absorption spectrometry.Waste Manag. 2012 May;32(5):944-8. doi: 10.1016/j.wasman.2011.12.001. Epub 2011 Dec 27. Waste Manag. 2012. PMID: 22206740
-
Improving the work environment in the fluorescent lamp recycling sector by optimizing mercury elimination.Waste Manag. 2018 Jun;76:250-260. doi: 10.1016/j.wasman.2018.02.037. Epub 2018 Mar 2. Waste Manag. 2018. PMID: 29496382 Review.
-
Mercury risk from fluorescent lamps in China: current status and future perspective.Environ Int. 2012 Sep;44:141-50. doi: 10.1016/j.envint.2012.01.006. Epub 2012 Feb 7. Environ Int. 2012. PMID: 22321538 Review.
Cited by
-
Mercury exposure and children's health.Curr Probl Pediatr Adolesc Health Care. 2010 Sep;40(8):186-215. doi: 10.1016/j.cppeds.2010.07.002. Curr Probl Pediatr Adolesc Health Care. 2010. PMID: 20816346 Free PMC article. Review.
-
Reduction of selenite to elemental selenium nanoparticles by activated sludge.Environ Sci Pollut Res Int. 2016 Jan;23(2):1193-202. doi: 10.1007/s11356-015-5138-7. Epub 2015 Sep 9. Environ Sci Pollut Res Int. 2016. PMID: 26351196
-
In Situ Room Temperature Electron-Beam Driven Graphene Growth from Hydrocarbon Contamination in a Transmission Electron Microscope.Materials (Basel). 2018 May 26;11(6):896. doi: 10.3390/ma11060896. Materials (Basel). 2018. PMID: 29861457 Free PMC article. Review.
-
Biomimetic mercury immobilization by selenium functionalized polyphenylene sulfide fabric.Nat Commun. 2024 Feb 12;15(1):1292. doi: 10.1038/s41467-024-45486-7. Nat Commun. 2024. PMID: 38346957 Free PMC article.
-
Amorphous Molybdenum Selenide Nanosheet as an Efficient Trap for the Permanent Sequestration of Vapor-Phase Elemental Mercury.Adv Sci (Weinh). 2019 Aug 14;6(20):1901410. doi: 10.1002/advs.201901410. eCollection 2019 Oct 16. Adv Sci (Weinh). 2019. PMID: 31637169 Free PMC article.
References
-
- Jang M.; Hong S. M.; Park J. K. Characterization and recovery of mercury from spent fluorescent lamps. Waste Manage. 2005, 25, 5–14. - PubMed
-
- EPA Final Rule, Hazardous Waste Management System, Modification of the HazardousWaste Program, Hazardous Waste Lamps, Federal Register, 1999;. Vol. 64, No. 128.
-
- Raposo C.; Windmoller C. C.; Junior W. A. D. Mercury speciation in fluorescent lamps by hermal release analysis. Waste Manage. 2003, 23, 879–886. - PubMed
-
- Thaler E. G.; Wilson R. H.; Doughty D. A.; Beers W. W. Measurement of mercury bound in the glass envelope during operation of fluorescent lamps. J. Electrochem. Soc. 1995, 142 (6), 1968–1970.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

