Detection of VM55599 and preparaherquamide from Aspergillus japonicus and Penicillium fellutanum: biosynthetic implications
- PMID: 18754595
- PMCID: PMC2834492
- DOI: 10.1021/np800292n
Detection of VM55599 and preparaherquamide from Aspergillus japonicus and Penicillium fellutanum: biosynthetic implications
Abstract
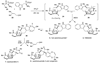
The secondary metabolites VM55599 (4) and preparaherquamide (5) have been identified by LC-MS(n) analysis as natural metabolites in cultures of Penicillium fellutanum, whereas preparaherquamide has been identified only in cultures of Aspergillus japonicus. In accord with a previous proposal, the identification of both metabolites, which have a diastereomeric relationship, provides indirect support for a unified biosynthetic scheme.
Figures





References
-
- Yamazaki M, Okuyama E, Kobayashi M, Inoue H. Tetrahedron Lett. 1981;22:135–136.
- Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L. J. Antibiot. 1990;43:1375–1379. - PubMed
- Liesch JM, Wichmann CF. J. Antibiot. 1990;43:1380–1386. - PubMed
- Blanchflower SE, Banks RM, Everett JR, Manger BR, Reading C. J. Antibiot. 1991;44:492–497. - PubMed
-
- Qian-Cutrone J, Huang S, Shu Y-Z, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q. J. Am. Chem. Soc. 2002;124:14556–14557. - PubMed
- Qian-Cutrone J, Krampitz K, Shu Y-Z, Chang LP. U.S. Patent 6,291,461. 2001.
-
- Birch AJ, Wright JJ. J. Chem. Soc. Chem. Commun. 1969:644–645.
- Birch AJ, Wright JJ. Tetrahedron. 1970;26:2329–2344. - PubMed
- Birch AJ, Russell RA. Tetrahedron. 1972;28:2999–3008.
- Bird BA, Remaley AT, Campbell IM. Appl. Environ. Microbiol. 1981;42:521–525. - PMC - PubMed
- Bird BA, Campbell IM. Appl. Environ. Microbiol. 1982;43:345–348. - PMC - PubMed
- Robbers JE, Straus JW. Lloydia. 1975;38:355–356. - PubMed
- Paterson RRM, Hawksworth DL. Trans. Br. Mycol. Soc. 1985;85:95–100.
- Wilson BJ, Yang DTC, Harris TM. Appl. Microbiol. 1973;26:633–635. - PMC - PubMed
- Coetzer J. Acta Crystallogr. 1974;B30:2254–2256.
-
- Polonsky J, Merrien M-A, Prange T, Pascard C. J. Chem. Soc. Chem. Comm. 1980:601–602.
- Prange T, Buillion M-A, Vuilhorgne M, Pascard C, Polonsky J. Tetrahedron Lett. 1980;22:1977–1980.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

