Self-assembly of brome mosaic virus capsids: insights from shorter time-scale experiments
- PMID: 18754598
- PMCID: PMC2753409
- DOI: 10.1021/jp802498z
Self-assembly of brome mosaic virus capsids: insights from shorter time-scale experiments
Abstract
An amended kinetic model for the self-assembly of empty capsids of brome mosaic virus is proposed. The model has been modified to account for a new feature in the assembly kinetics revealed by time-course light scattering experiments at higher temporal resolution than previously attempted. To be able to simulate the sharp takeoff from the initial lag phase to the growth phase in the kinetic curves, a monomer activation step was proposed.
Figures




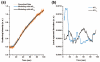
Similar articles
-
A theory for viral capsid assembly around electrostatic cores.J Chem Phys. 2009 Mar 21;130(11):114902. doi: 10.1063/1.3086041. J Chem Phys. 2009. PMID: 19317561 Free PMC article.
-
Electrophoretic mobilities of a viral capsid, its capsid protein, and their relation to viral assembly.J Phys Chem B. 2014 Feb 27;118(8):1984-9. doi: 10.1021/jp407379t. Epub 2014 Feb 14. J Phys Chem B. 2014. PMID: 24467401
-
Unravelling the Stability and Capsid Dynamics of the Three Virions of Brome Mosaic Virus Assembled Autonomously In Vivo.J Virol. 2020 Mar 31;94(8):e01794-19. doi: 10.1128/JVI.01794-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31996436 Free PMC article.
-
The brome mosaic virus 3' untranslated sequence regulates RNA replication, recombination, and virion assembly.Virus Res. 2015 Aug 3;206:46-52. doi: 10.1016/j.virusres.2015.02.007. Epub 2015 Feb 14. Virus Res. 2015. PMID: 25687214 Review.
-
The interplay between capsid dynamics and pathogenesis in tripartite bromoviruses.Curr Opin Virol. 2021 Apr;47:45-51. doi: 10.1016/j.coviro.2020.12.005. Epub 2021 Jan 28. Curr Opin Virol. 2021. PMID: 33517133 Review.
Cited by
-
Physical, chemical, and synthetic virology: Reprogramming viruses as controllable nanodevices.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019 May;11(3):e1545. doi: 10.1002/wnan.1545. Epub 2018 Nov 8. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019. PMID: 30411529 Free PMC article. Review.
-
Speed limits of protein assembly with reversible membrane localization.J Chem Phys. 2021 May 21;154(19):194101. doi: 10.1063/5.0045867. J Chem Phys. 2021. PMID: 34240891 Free PMC article.
-
Modeling Viral Capsid Assembly.Adv Chem Phys. 2014;155:1-68. doi: 10.1002/9781118755815.ch01. Adv Chem Phys. 2014. PMID: 25663722 Free PMC article.
-
Using Markov state models to study self-assembly.J Chem Phys. 2014 Jun 7;140(21):214101. doi: 10.1063/1.4878494. J Chem Phys. 2014. PMID: 24907984 Free PMC article.
-
Mechanisms of virus assembly.Annu Rev Phys Chem. 2015 Apr;66:217-39. doi: 10.1146/annurev-physchem-040214-121637. Epub 2014 Dec 17. Annu Rev Phys Chem. 2015. PMID: 25532951 Free PMC article. Review.
References
-
- Hiebert E, Bancroft JB. Factors Affecting the Assembly of Some Spherical Viruses. Virology. 1969;39:296–311. - PubMed
-
- Pfeiffer P, Hirth L. Aggregation States of Brome Mosaic Virus Protein. Virology. 1974;61:160–167. - PubMed
-
- Chen XJS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of Small Virus-Like Particles Assembled from the L1 Protein of Human Papillomavirus 16. Mol. Cell. 2000;5(3):557–567. - PubMed
-
- Fuller MT, King J. Assembly Invitro of Bacteriophage-P22 Procapsids from Purified Coat and Scaffolding Subunits. J. Mol. Biol. 1982;156(3):633–665. - PubMed
-
- Prevelige PE, Thomas D, King J. Scaffolding Protein Regulates the Polymerization of P22 Coat Subunits into Icosahedral Shells Invitro. J. Mol. Biol. 1988;202(4):743–757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

