Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus
- PMID: 18754811
- PMCID: PMC2632688
- DOI: 10.1111/j.1365-2567.2008.02895.x
Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus
Erratum in
- Immunology. 2009 Mar;126(3):446
Abstract
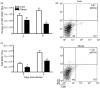
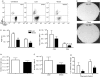
There is a clear link between obesity and metabolic disorders; however, little is known about the effect of obesity on immune function, particularly during an infection. We have previously reported that diet-induced obese mice are more susceptible to morbidity and mortality during influenza infection than lean mice. Obese mice displayed aberrant innate immune responses characterized by minimal induction of interferon (IFN)-alpha/beta, delayed expression of pro-inflammatory cytokines and chemokines, and impaired natural killer cell cytotoxicity. To further examine the abnormal immune response of diet-induced obese mice, we analysed the cellularity of their lungs during influenza virus infection. We found delayed mononuclear cell entry with a marked decrease in dendritic cells (DCs) throughout the infection. Given the critical role of the DC in activating the cell-mediated immune response, we also analysed the functional capacity of DCs from obese mice. We found that, while obesity did not interfere with antigen uptake and migration, it did impair DC antigen presentation. This was probably attributable to an altered cytokine milieu, as interleukin (IL)-2, IL-12, and IL-6 were differentially regulated in the obese mice. Overall, this did not impact the total number of virus-specific CD8(+) T cells that were elicited, but did affect the number and frequency of CD3(+) and CD8(+) T cells in the lung. Thus, obesity interferes with cellular responses during influenza infection, leading to alterations in the T-cell population that ultimately may be detrimental to the host.
Figures




References
-
- Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. - PubMed
-
- Perlemuter GBA, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–69. - PubMed
-
- Norris S, Provo B, Stotts N. Physiology of wound healing and risk factors that impede the healing process. AACN Clin Issues Crit Care Nurs. 1990;1:545–52. - PubMed
-
- Massie J, Heller J, Abitbol J, McPherson D, Garfin S. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

